Key Points
CALR mutations occur in half of JAK2 and MPL wt patients with ET and associate with some distinctive phenotypic traits.
Patients with ET harboring CALR mutations are at significantly lower risk of thrombosis compared with JAK2- and MPL-mutated patients.
Abstract
Mutations in the calreticulin (CALR) gene were recently discovered in patients with essential thrombocythemia (ET) lacking the JAK2V617F and MPLW515 mutations, but no information is available on the clinical correlates. In this series, CALR mutations were found in 15.5% of 576 World Health Organization–defined ET patients, accounting for 48.9% of JAK2 and MPL wild-type (wt) patients. CALR-mutated patients were preferentially male and showed higher platelet count and lower hemoglobin and leukocyte count compared with JAK2- and MPL-mutated patients. Patients carrying the CALR mutation had a lower risk of thrombosis than JAK2- and MPL-mutated patients; of interest, their risk was superimposable to patients who were wt for the above mutations. CALR mutation had no impact on survival or transformation to post-ET myelofibrosis. Genotyping for CALR mutations represents a novel useful tool for establishing a clonal myeloproliferative disorder in JAK2 and MPL wt patients with thrombocytosis and may have prognostic and therapeutic relevance.
Introduction
Unlike polycythemia vera (PV), where virtually all the patients harbor JAK2 mutations (JAK2V617F in >95% and JAK2 exon 12 mutations in ∼2-4%), only 50% to 60% of essential thrombocythemia (ET)1-3 and primary myelofibrosis (PMF)4-6 patients have JAK2V617F mutation (JAK2+). An additional 3% to 5% of ET and PMF subjects present MPL mutations at codon 515 (MPL+).7-9 JAK2 and MPL mutations represent major diagnostic criteria in the 2008 World Health Organization (WHO) classification of chronic myeloproliferative neoplasms (MPN).10,11 All these mutations result in the abnormal activation of the Janus kinase/signal transducer and activator of transcription signaling pathway that represents a hallmark of MPN and a target for therapy.12 Recently, mutations at exon 9 of CALR, the gene encoding calreticulin, an endoplasmic reticulum Ca2+-binding chaperone, were discovered in 50% to 70% of patients with ET and PMF (CALR+) who were wild type (wt) for JAK2 and MPL.13,14 The mechanisms by which CALR mutations produce a myeloproliferative phenotype are unknown.
The aim of this study was to describe the prevalence, characteristics, and clinical and laboratory features associated with CALR mutations in a large population of patients with WHO-defined ET.
Study design
The study involved 576 patients with a diagnosis of ET fulfilling the 2008 WHO criteria who were followed at the Hematology Department, University of Florence. They had a stored sample of granulocyte DNA collected at diagnosis or within 3 years. Patients had provided an informed written consent in accordance with the Declaration of Helsinki for the use of DNA for investigational purposes. The Ethical Committee was that of the Azienda Ospedaliero-Universitaria Careggi in Florence. The JAK2V617F and MPLW515L/K mutations were assessed by real-time quantitative polymerase chain reaction15,16 and also by high-resolution melting analysis followed by bidirectional Sanger sequencing for MPL.17 Mutations in exon 9 of CALR were assessed by bidirectional sequencing.13
Patient characteristics reported in Table 1 were obtained at diagnosis. Splenomegaly was defined as a palpable organ below the left costal margin. Major thrombosis and bleeding, at diagnosis and/or in the 2 preceding years and/or anytime during follow-up, were recorded when objectively documented18 and according to standard definitions.19 Microvessel symptoms consisted of erythromelalgia and recurrent episodes of otherwise unexplained blurred vision, tinnitus, paresthesia, and headache. Constitutional symptoms included fever, unintentional weight loss, and night sweats. Pruritus was recorded when described as a diffuse, recurrent itching exacerbated by water contact. Evolution to post-ET myelofibrosis (PET-MF) and acute leukemia (AL) was diagnosed following the International Working Group for Myeloproliferative Neoplasms Research and Treatment and WHO criteria, respectively.11,20 Patients were treated according to current recommendations21 ; cytoreduction was hydroxyurea in >90% of high-risk patients.
Laboratory and clinical characteristics of CALR mutant patients compared with JAK2V617F or MPLW515 mutant patients and patients who were wt for the 3 mutations
| . | CALR+ . | JAK2 V617F+ . | MPL W515+ . | CALR, JAK2, MPL wt . | P value . | ||
|---|---|---|---|---|---|---|---|
| CALR+ vs JAK2 V617F+ . | CALR+ vs MPL W515+ . | CALR+ vs CALR, JAK2, MPL wt . | |||||
| Number of patients (%) | 89 (15.5) | 369 (64.1) | 25 (4.3) | 93 (16.1) | — | — | — |
| Male, no. (%) | 53 (59.5) | 117 (31.7) | 6 (24.0) | 18 (19.4) | <0.0001 | 0.002 | <0.0001 |
| Age, years | 54.7 (13-88) | 61 (15-93) | 54 (22.89) | 53 (15-87) | 0.04 | 0.997 | 0.519 |
| Leukocyte count (×109/L) | 8.1 (3.5-26.0) | 8.9 (4.2-35.0) | 8.4 (4.5-16.6) | 8.3 (4-16.8) | 0.001 | 0.834 | 0.367 |
| Hemoglobin (g/L) | 138 (106-173) | 145 (102-173) | 136 (110-160) | 136 (106-164) | <0.0001 | 0.315 | 0.380 |
| Hematocrit (%) | 41.2 (35.9-49.4) | 43.8 (31.4-53.6) | 41.2 (32.8-50) | 41.0 (31.3-51.5) | <0.0001 | 0.887 | 0.893 |
| Platelet count (×109/L) | 866 (504-2348) | 726 (455-1881) | 898 (607-2000) | 697 (482-1659) | <0.0001 | 0.385 | <0.0001 |
| Lactate dehydrogenase (U/L) | 320 (142-725) | 288 (102-1178) | 365 (254-570) | 268 (137-554) | 0.307 | 0.665 | <0.01 |
| Splenomegaly, no. (%) | 24 (27.0) | 91 (24.7) | 9 (36.0) | 9 (9.7) | 0.661 | 0.416 | 0.004 |
| Pruritus, no. (%) | 5 (5.6) | 32 (8.7) | 1 (4.0) | 9 (9.7) | 0.260 | 0.847 | 0.228 |
| Constitutional symptoms, no. (%) | 1 (1.1) | 19 (5.1) | 1 (1.2) | 6 (6.5) | 0.120 | 0.577 | 0.078 |
| Major thrombosis, no. (%) | 12 (13.5) | 111 (30.1) | 10 (40.0) | 15 (16.1) | 0.011 | 0.012 | 0.894 |
| Microvessel symptoms, no. (%) | 22 (24.7) | 101 (27.4) | 14 (56.0) | 20 (21.5) | 0.604 | 0.003 | 0.674 |
| Major hemorrhage, no. (%) | 4 (4.5) | 17 (4.6) | 4 (16.0) | 3 (3.3) | 0.906 | 0.067 | 0.587 |
| Progression to PET-MF, no. (%) | 4 (4.5) | 12 (3.3) | 2 (8.0) | 1 (1.1) | 0.458 | 0.563 | 0.128 |
| Progression to PV, no. (%) | 0 | 5 (1.4) | 0 | 0 | 0.294 | — | — |
| Progression to AL, no. (%) | 0 | 2 (0.5) | 1 (4.0) | 1 (1.1) | 0.507 | 0.071 | 0.349 |
| Deceased (n = 70) (%) | 10 (11.2) | 49 (13.3) | 4 (16.0) | 7 (7.5) | 0.598 | 0.515 | 0.414 |
| Cytoreductive therapy, no. (%) | 50 (62.5) | 220 (60.8) | 19 (76.0) | 40 (46.0) | 0.440 | 0.160 | 0.023 |
| . | CALR+ . | JAK2 V617F+ . | MPL W515+ . | CALR, JAK2, MPL wt . | P value . | ||
|---|---|---|---|---|---|---|---|
| CALR+ vs JAK2 V617F+ . | CALR+ vs MPL W515+ . | CALR+ vs CALR, JAK2, MPL wt . | |||||
| Number of patients (%) | 89 (15.5) | 369 (64.1) | 25 (4.3) | 93 (16.1) | — | — | — |
| Male, no. (%) | 53 (59.5) | 117 (31.7) | 6 (24.0) | 18 (19.4) | <0.0001 | 0.002 | <0.0001 |
| Age, years | 54.7 (13-88) | 61 (15-93) | 54 (22.89) | 53 (15-87) | 0.04 | 0.997 | 0.519 |
| Leukocyte count (×109/L) | 8.1 (3.5-26.0) | 8.9 (4.2-35.0) | 8.4 (4.5-16.6) | 8.3 (4-16.8) | 0.001 | 0.834 | 0.367 |
| Hemoglobin (g/L) | 138 (106-173) | 145 (102-173) | 136 (110-160) | 136 (106-164) | <0.0001 | 0.315 | 0.380 |
| Hematocrit (%) | 41.2 (35.9-49.4) | 43.8 (31.4-53.6) | 41.2 (32.8-50) | 41.0 (31.3-51.5) | <0.0001 | 0.887 | 0.893 |
| Platelet count (×109/L) | 866 (504-2348) | 726 (455-1881) | 898 (607-2000) | 697 (482-1659) | <0.0001 | 0.385 | <0.0001 |
| Lactate dehydrogenase (U/L) | 320 (142-725) | 288 (102-1178) | 365 (254-570) | 268 (137-554) | 0.307 | 0.665 | <0.01 |
| Splenomegaly, no. (%) | 24 (27.0) | 91 (24.7) | 9 (36.0) | 9 (9.7) | 0.661 | 0.416 | 0.004 |
| Pruritus, no. (%) | 5 (5.6) | 32 (8.7) | 1 (4.0) | 9 (9.7) | 0.260 | 0.847 | 0.228 |
| Constitutional symptoms, no. (%) | 1 (1.1) | 19 (5.1) | 1 (1.2) | 6 (6.5) | 0.120 | 0.577 | 0.078 |
| Major thrombosis, no. (%) | 12 (13.5) | 111 (30.1) | 10 (40.0) | 15 (16.1) | 0.011 | 0.012 | 0.894 |
| Microvessel symptoms, no. (%) | 22 (24.7) | 101 (27.4) | 14 (56.0) | 20 (21.5) | 0.604 | 0.003 | 0.674 |
| Major hemorrhage, no. (%) | 4 (4.5) | 17 (4.6) | 4 (16.0) | 3 (3.3) | 0.906 | 0.067 | 0.587 |
| Progression to PET-MF, no. (%) | 4 (4.5) | 12 (3.3) | 2 (8.0) | 1 (1.1) | 0.458 | 0.563 | 0.128 |
| Progression to PV, no. (%) | 0 | 5 (1.4) | 0 | 0 | 0.294 | — | — |
| Progression to AL, no. (%) | 0 | 2 (0.5) | 1 (4.0) | 1 (1.1) | 0.507 | 0.071 | 0.349 |
| Deceased (n = 70) (%) | 10 (11.2) | 49 (13.3) | 4 (16.0) | 7 (7.5) | 0.598 | 0.515 | 0.414 |
| Cytoreductive therapy, no. (%) | 50 (62.5) | 220 (60.8) | 19 (76.0) | 40 (46.0) | 0.440 | 0.160 | 0.023 |
Hematologic and clinical information was collected at diagnosis; information regarding major thrombosis and hemorrhage included events at diagnosis, in the 2 preceding years, and during follow-up. Cytoreduction means that the patient received cytoreductive drugs (in >90% of cases, this was hydroxyurea) during the course of the disease at the physician’s discretion, based on conventional criteria. Unless otherwise indicated, values are reported as median (range). Statistically significant differences are shown in bold.
Statistical analysis was performed with SPSS software. Patient characteristics were compared with the use of the χ2 test or Fisher's exact test for categorical variables and the t-test or nonparametric test for continuous variables. The significance level was P < .05 in 2-sided tests. Survival estimates were obtained with the Kaplan-Meier method; the hazard ratio was determined using a Cox proportional hazards model.
Results and discussion
In the entire patient series, the median age was 58.1 years (range, 13-93 years); 194 subjects (33.7%) were male. Median follow-up was 71.9 months (range, 2-257 months); 70 patients (12.1%) died a median of 53.9 months (range, 2-249 months) after diagnosis. Nineteen patients (3.3%) progressed to PET-MF after a median of 122 months (range, 19-248 months); transformation to PV was documented in 5 cases (0.9%), and 4 patients (0.7%) evolved to AL after a median of 117.7 months (range, 56-250 months). We found 89 patients (15.5% of total) harboring exon 9 CALR mutations. CALR mutations were represented by insertions and deletions, as previously reported.13,14 Deletions (60.7%) occurred more frequently than insertions (39.3%); the most common deletion was del367fs46 (37.0%), and ins385fs47 (71.4%) was the most common among insertions. JAK2V617F and MPLW515 mutations occurred in 64.1% (n = 369) and 4.3% (n = 25) of patients. CALR+ patients accounted for 48.9% of JAK2 and MPL wt patients (n = 182); 93 patients (16.1% of total) were wt for the 3 mutations considered.
We compared hematological and clinical characteristics of the patients who were categorized according to their JAK2V617F, MPLW515, and CALR genotype (Table 1). CALR+ patients were younger than JAK2+ and no different from MPL+ and wt; a striking male predominance was found among CALR+ (59.5%) compared with JAK2+ (31.7%; P < .001), MPL+ (24.0%, P = .002), and wt (19.4%; P < .001) patients. Influence of gender on JAK2V617F allele burden,22 disease class, and vascular complications23 is well documented, and current data add to the understanding of the role of host variations for the expression of the MPN phenotype.24
The leukocyte count, hemoglobin, and hematocrit level were lower in CALR+ compared with JAK2+ (P = .001, P < .0001, and P < .0001, respectively) and were similar to MPL+ and wt patients; on the other hand, the platelet count was higher in CALR+ than in JAK2+ and wt patients (P < .0001 for both), but comparable to MPL+ patients who also differed significantly from JAK2+ (P < .001). Lactate dehydrogenase level was lower in wt compared with patients with any mutation (P < .001). Constitutional symptoms and pruritus were similarly represented in the different groups; a palpable spleen was less common in wt patients compared with those harboring any mutation (P < .01). The proportion of CALR+ patients who received cytoreduction was similar to JAK2+ and MPL+ and lower than wt patients (Table 1). Overall, these findings indicate that CALR+ patients, similar to MPL+ patients, present a phenotype associated with preferential expansion of the megakaryocytic lineage compared with favored erythropoiesis in JAK2+ patients.
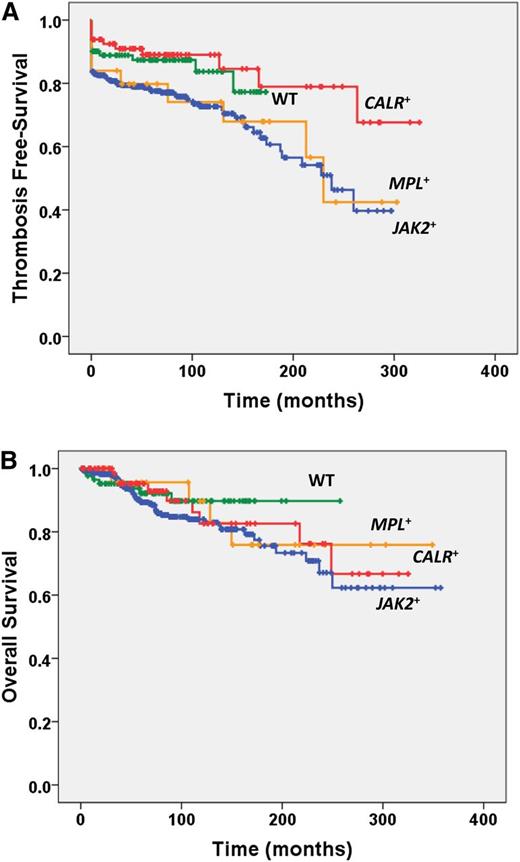
Major cardiovascular events occurred in 30.1%, 40.0%, 13.5%, and 16.1% of the JAK2+, MPL+, CALR+, and wt patients, respectively; the difference was statistically significant when comparing CALR+ vs JAK2+ and MPL+ patients (P = .01 for both). On the other hand, microvessel symptoms were more represented among MPL+ patients (P < .01 compared with the other groups). The thrombosis-free survival was significantly longer in CALR+ and wt patients compared with JAK2+ and MPL+ (P = .008; Figure 1A). The cumulative incidence of thrombosis at 10 years was 5.12% (95% confidence interval [CI], 1.6-15.2) in CALR+, 14.54% (95% CI, 10.0-20.8) in JAK2+, 19.46% (95% CI, 7.6-44.6) in MPL+, and 8.17% (95% CI, 2.7-23.3) in wt patients. Taking wt patients as the reference population, the HR for thrombosis was 0.74 (95% CI, 0.33-1.00) for CALR+, 1.78 (95% CI, 1.06-3.18) for JAK2+, and 1.65 (95% CI, 1.7-3.92) for MPL+ patients. There was a trend toward more frequent hemorrhages in MPL+ compared with all other patients. The median survival was not reached in any group, and Kaplan-Meier estimates of survival did not show significant differences (Figure 1B). CALR+ patients were preferentially distributed in the lower-risk category of the thrombosis score, the International Prognostic Score in Essential Thrombocythemia (IPSET), and the IPSET-thrombosis score compared with JAK2+ (and MPL+ for IPSET-thrombosis) (supplemental Table 1 available on the Blood website). Overall, these data indicate that CALR+ patients are less prone to thrombotic events compared with JAK2+ and MPL+; of note, their risk was similar to patients lacking any mutations.
Overall Survival and Thrombosis-free survival according to mutational status. Kaplan-Meier estimate of (A) thrombosis-free survival and (B) overall survival in patients who were categorized according to their mutational status (JAK2V617+, MPLW515+, CALR+, or wt for the above mutations).
Overall Survival and Thrombosis-free survival according to mutational status. Kaplan-Meier estimate of (A) thrombosis-free survival and (B) overall survival in patients who were categorized according to their mutational status (JAK2V617+, MPLW515+, CALR+, or wt for the above mutations).
Transformation to PET-MF occurred in 19 patients; the hazard ratio for PET-MF was similarly increased in CALR+ (2.36; 95% CI, 0.26-21.8), JAK2+ (2.21; 95% CI, 0.28-17.8), and MPL+ (2.50; 95% CI, 0.22-28.5) patients compared with wt, although, possibly due to a small number of events, this did not reach the significance level. Noteworthy, all 5 cases of transformation to PV occurred in the JAK2+ patients.
With the limitations imposed by its observational nature, which precludes any causal relationships inferences, results of the current study identified meaningful associations between the presence of CALR mutations and the phenotype of patients with ET. The findings that CALR-mutated patients are at lower risk of vascular events may have implications for risk stratification and management. Finally, our study underscores the importance of CALR genotyping for an accurate diagnosis of patients with thrombocytosis who lack the JAK2V617F and MPLW515 mutations.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Carobbio for helpful advice in statistical analysis.
This study was supported by a special grant from Associazione Italiana per la Ricerca sul Cancro “AIRC 5 per Mille” to Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative (AGIMM) (#1005). This work was also partially supported by Ministero della Università e Ricerca (FIRB project RBAP11CZLK and PRIN 2010NYKNS7).
Authorship
Contributions: P.G. and A.M.V. designed the study, analyzed the data, and wrote the manuscript; G.R. and C.M. performed molecular analysis and analyzed raw sequencing data; A. Pacilli, A. Pancrazzi, and T.F. contributed to molecular analysis; P.G., L.P., A.B., and A.M.V. contributed samples and clinical information; and all authors read the final version of the manuscript and agreed on its content. For a description of the AGIMM project and list of investigators, see www.progettoagimm.it.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paola Guglielmelli, Laboratorio Congiunto-MMPC, Department of Experimental and Clinical Medicine, University of Florence and Azienda Ospedaliera-Universitaria Careggi, Largo Brambilla 3, 50134 Florence, Italy; e-mail: paola.guglielmelli@unifi.it; and Alessandro M. Vannucchi, Laboratorio Congiunto-MMPC, Department of Experimental and Clinical Medicine, University of Florence and Azienda Ospedaliera-Universitaria Careggi, Largo Brambilla 3, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it.
References
Author notes
G.R., C.M., and P.G. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal