Key Points
Intensive BFM therapy is effective for HR childhood ALL if low MRD levels are achieved at the end of the induction/consolidation phase.
Childhood ALL with high MRD levels at the end of induction/consolidation phase has a poor prognosis despite intensive BFM therapy or HSCT.
Abstract
The outcome of high-risk (HR) acute lymphoblastic leukemia patients enrolled in the AIEOP-BFM ALL 2000 study in Italy is described. HR criteria were minimal residual disease (MRD) levels ≥10−3 at day 78 (MRD-HR), no complete remission (CR) at day 33, t(4;11) translocation, and prednisone poor response (PPR). Treatment (2 years) included protocol I, 3 polychemotherapy blocks, delayed intensification (protocol IIx2 or IIIx3), cranial radiotherapy, and maintenance. A total of 312 HR patients had a 5-year event-free survival (EFS) of 58.9% (standard error [SE] = 2.8) and an overall survival of 68.9% (SE = 2.6). In hierarchical order, EFS was 45.9% (4.4) in 132 MRD-HR patients, 41.2% (11.9) in 17 patients with no CR at day 33, 36.4% (14.5) in 11 patients with t(4;11), and 74.0% (3.6) in 152 HR patients only for PPR. No statistically significant difference was found for disease-free survival in patients with very HR features [MRD-HR, no CR at day 33, t(4;11) translocation], given hematopoietic stem cell transplantation (HSCT) (n = 66) or chemotherapy only (n = 88), after adjusting for waiting time to HSCT (5.7 months). Patients at HR only for PPR have a favorable outcome. MRD-HR is associated with poor outcome despite intensive treatment and/or HSCT and may qualify for innovative therapies. The study was registered at www.clinicaltrials.gov as #NCT00613457.
Introduction
In the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) and Berlin-Frankfurt-Münster (BFM) acute lymphoblastic leukemia (ALL) 2000 study, both the AIEOP and BFM used prospective evaluation of minimal residual disease (MRD) for patient stratification.1-4
In the studies AIEOP-ALL 91 and ALL-BFM 90, it was shown that conventional induction therapy (5 weeks) followed by 9 chemotherapy blocks and maintenance therapy led to poor results (5-year event-free survival [EFS] of 40%) in high-risk (HR) patients (approximately 15% of the ALL population), including infants and children with Philadelphia-positive ALL.5,6
In the subsequent AIEOP ALL 95 study, treatment strategy was based on conventional BFM therapy and intensified with 3 chemotherapy blocks and double-reinduction therapy and improved the outcome of HR patients, with a 5-year EFS of 53%.7,8 Similar results were obtained in the ALL-BFM 95 study substituting reinduction protocol II for 3 blocks.9
The 95 study’s therapeutic approach was thus adopted in the AIEOP-BFM ALL 2000 study, where MRD response was regarded as the most important prognostic factor.1-3,10,11 Stratification criteria were common for the AIEOP and BFM studies, but there were differences in the treatment of HR patients and the indications for hematopoietic stem cell transplantation (HSCT) that justify separate reporting. Therefore, results obtained in HR patients treated with chemotherapy or HSCT in the context of the AIEOP ALL 2000 study are reported here.
Methods
Patients
The AIEOP-BFM ALL 2000 study was approved by the ethical committees of all participating institutions. The study was conducted in accordance with the Declaration of Helsinki. From September 1, 2000, to July 31, 2006, a total of 1999 patients with Philadelphia-negative ALL between 1 and 18 years of age were diagnosed in one of the 41 participating study centers in Italy and were eligible to enter the AIEOP-BFM ALL 2000 study. Of these, 312 (15.6%) were stratified in the HR group on the basis of at least one of the following criteria: prednisone poor response (PPR) (patients with at least 1000 blast cells per μL peripheral blood after 7 days of prednisone monotherapy and 1 injection of intrathecal methotrexate [MTX]), failure to achieve complete remission (CR) after the first 5 weeks (day 33) of induction therapy (protocol IA), evidence of translocation t(4;11) upon prospective screening, and MRD level ≥ 10−3 (MRD level 10−3 indicates the interval of values between ≥5 × 10−4 and <5 × 10−3) at day 78 bone marrow (BM) aspirate, after induction and consolidation therapy (protocol IA and IB).2,3
Diagnostic studies
The diagnosis of ALL was confirmed using cytomorphology and cytochemistry (French-American-British criteria) when ≥25% of lymphoblastic cells were present in the BM. For all samples, flow-cytometric immunophenotyping and screening of ETV6-RUNX1, BCR/ABL, and MLL/AF4 fusion transcripts were performed.
CR was defined as the absence of physical signs of leukemia or detectable leukemia cells on blood smears, a BM with active hematopoiesis and <5% leukemia blast cells, and normal cerebrospinal fluid. Patients who did not achieve CR at the end of induction phase IA were treated with phase IB of protocol I followed by 3 HR blocks; resistance was defined as failure to achieve CR by the end of the third HR block (see Table 1 for protocol details).
AIEOP-BFM ALL 2000 treatment phases for AIEOP HR patients
| Drug/administration route . | Daily dose (mg/m2 ) . | Day . |
|---|---|---|
| Prephase | ||
| Prednisone/po-IV | 60 | 1-7 |
| MTX/IT | By age* | 1 |
| Induction: protocol IA | ||
| Vincristine/IV | 1.5 (max 2 mg) | 8, 15, 22, 29 |
| Prednisone/po-IV (®†) | 60 | 8-28 then tapered |
| or Dexamethasone/po-IV (®†) | 10 | 8-28 then tapered |
| Daunorubicin/IV | 30 | 8, 15, 22, 29 |
| l-Asparaginase/IM | 5000 IU/m2 | 12, 15, 18, 21, 24, 27, 30, 33 |
| MTX/IT | By age* | 1, 15, 29|| |
| Consolidation: protocol IB | ||
| Cyclophosphamide/IV | 1000 | 36, 64 |
| Mercaptopurine/po | 60 | 36-63 |
| Cytarabine/IV-SC | 75 | 38-41, 45-48, 52-55, 59-62 |
| MTX/IT | By age* | 38, 52 |
| HR block 1 | ||
| Dexamethasone/po-IV | 20 | 1-5 |
| Vincristine/IV | 1.5 (max 2 mg) | 1, 6 |
| HD Cytarabine/IV | 2000 × 2 | 5 |
| MTX/IV | 5000 | 1 |
| Leucovorin rescue‡ | 15 mg/m2/dose | 42, 48, 54 h after start HD-MTX |
| Cyclophosphamide/IV | 200 (q12h × 5) | 2-4 |
| l-Asparaginase/IM | 10 000 IU/m2 | 6 |
| MTX/IT | By age* | 1 |
| HR block 2 | ||
| Dexamethasone/po-IV | 20 | 1-5 |
| Vindesine/IV | 3 | 1, 6 |
| Daunorubicin/IV | 30 | 5 |
| MTX/IV | 5000 | 1 |
| Leucovorin rescue‡ | 15 mg/m2/dose | 42, 48, 54 h after start HD-MTX |
| Ifosphamide/IV | 800 (q12h × 5) | 2-4 |
| l-Asparaginase/IM | 10 000 IU/m2 | 6 |
| MTX/IT | By age* | 1 |
| HR block 3 | ||
| Dexamethasone/po-IV | 20 | 1-5 |
| HD Cytarabine/IV | 2000 (q 12 h × 4) | 1, 2 |
| Etoposide/IV | 100 (q 12 h × 5) | 3-5 |
| l-Asparaginase/IM | 10 000 IU/m2 | 6 |
| MTX/IT | By age* | 5 |
| Reinduction (protocol II) | ||
| Dexamethasone/po- IV§ | 10 | 1-21 then tapered |
| Vincristine/IV | 1.5 (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin/IV | 25 | 8, 15, 22, 29 |
| l-Asparaginase/IM | 10 000 IU/m2 | 8, 11, 15, 18 |
| 6-Thioguanine/po | 60 | 36-49 |
| Cyclophosphamide/IV | 1000 | 36 |
| Cytarabine/IV-SC | 75 | 38-41, 45-47 |
| MTX/IT | By age* | 38, 45 |
| Cranial irradiation¶ | By age | |
| Reinduction (protocol III) | ||
| Dexamethasone/po-IV | 10 | 1-14 then tapered |
| Vincristine/ IV | 1.5 (max 2 mg) | 1, 8 |
| Doxorubicin/IV | 30 | 1, 8 |
| l-Asparaginase/IM | 10 000 IU/m2 | 1, 4, 8, 11 |
| 6-Thioguanine/po | 60 | 15-28 |
| Cyclophosphamide/IV | 500 | 15 |
| Cytarabine/IV-SC | 75 | 17-20, 24-27 |
| MTX/IT | By age* | 17, 24 |
| Cranial irradiation¶ | By age | |
| Interim maintenance | ||
| Mercaptopurine/po | 50 | Daily |
| MTX/po | 20 | Weekly |
| Continuation phase‡ | ||
| Mercaptopurine/po | 50# | Daily |
| MTX/po | 20# | Weekly |
| Drug/administration route . | Daily dose (mg/m2 ) . | Day . |
|---|---|---|
| Prephase | ||
| Prednisone/po-IV | 60 | 1-7 |
| MTX/IT | By age* | 1 |
| Induction: protocol IA | ||
| Vincristine/IV | 1.5 (max 2 mg) | 8, 15, 22, 29 |
| Prednisone/po-IV (®†) | 60 | 8-28 then tapered |
| or Dexamethasone/po-IV (®†) | 10 | 8-28 then tapered |
| Daunorubicin/IV | 30 | 8, 15, 22, 29 |
| l-Asparaginase/IM | 5000 IU/m2 | 12, 15, 18, 21, 24, 27, 30, 33 |
| MTX/IT | By age* | 1, 15, 29|| |
| Consolidation: protocol IB | ||
| Cyclophosphamide/IV | 1000 | 36, 64 |
| Mercaptopurine/po | 60 | 36-63 |
| Cytarabine/IV-SC | 75 | 38-41, 45-48, 52-55, 59-62 |
| MTX/IT | By age* | 38, 52 |
| HR block 1 | ||
| Dexamethasone/po-IV | 20 | 1-5 |
| Vincristine/IV | 1.5 (max 2 mg) | 1, 6 |
| HD Cytarabine/IV | 2000 × 2 | 5 |
| MTX/IV | 5000 | 1 |
| Leucovorin rescue‡ | 15 mg/m2/dose | 42, 48, 54 h after start HD-MTX |
| Cyclophosphamide/IV | 200 (q12h × 5) | 2-4 |
| l-Asparaginase/IM | 10 000 IU/m2 | 6 |
| MTX/IT | By age* | 1 |
| HR block 2 | ||
| Dexamethasone/po-IV | 20 | 1-5 |
| Vindesine/IV | 3 | 1, 6 |
| Daunorubicin/IV | 30 | 5 |
| MTX/IV | 5000 | 1 |
| Leucovorin rescue‡ | 15 mg/m2/dose | 42, 48, 54 h after start HD-MTX |
| Ifosphamide/IV | 800 (q12h × 5) | 2-4 |
| l-Asparaginase/IM | 10 000 IU/m2 | 6 |
| MTX/IT | By age* | 1 |
| HR block 3 | ||
| Dexamethasone/po-IV | 20 | 1-5 |
| HD Cytarabine/IV | 2000 (q 12 h × 4) | 1, 2 |
| Etoposide/IV | 100 (q 12 h × 5) | 3-5 |
| l-Asparaginase/IM | 10 000 IU/m2 | 6 |
| MTX/IT | By age* | 5 |
| Reinduction (protocol II) | ||
| Dexamethasone/po- IV§ | 10 | 1-21 then tapered |
| Vincristine/IV | 1.5 (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin/IV | 25 | 8, 15, 22, 29 |
| l-Asparaginase/IM | 10 000 IU/m2 | 8, 11, 15, 18 |
| 6-Thioguanine/po | 60 | 36-49 |
| Cyclophosphamide/IV | 1000 | 36 |
| Cytarabine/IV-SC | 75 | 38-41, 45-47 |
| MTX/IT | By age* | 38, 45 |
| Cranial irradiation¶ | By age | |
| Reinduction (protocol III) | ||
| Dexamethasone/po-IV | 10 | 1-14 then tapered |
| Vincristine/ IV | 1.5 (max 2 mg) | 1, 8 |
| Doxorubicin/IV | 30 | 1, 8 |
| l-Asparaginase/IM | 10 000 IU/m2 | 1, 4, 8, 11 |
| 6-Thioguanine/po | 60 | 15-28 |
| Cyclophosphamide/IV | 500 | 15 |
| Cytarabine/IV-SC | 75 | 17-20, 24-27 |
| MTX/IT | By age* | 17, 24 |
| Cranial irradiation¶ | By age | |
| Interim maintenance | ||
| Mercaptopurine/po | 50 | Daily |
| MTX/po | 20 | Weekly |
| Continuation phase‡ | ||
| Mercaptopurine/po | 50# | Daily |
| MTX/po | 20# | Weekly |
IM, intramuscular; IT, intrathecal; po, by mouth; Pred, prednisone; SC, subcutaneous.
Age-adjusted doses of intrathecal MTX: ≥1 and <2 y: 8 mg; ≥2 and <3 y: 10 mg; ≥3 y: 12 mg
According to first randomization (®).
Leucovorin rescue: 7.5-mg/m2 dose for levorotatory compound given at hours 42, 48, and 54 for HD-MTX 5 g/m2.
Dexamethasone in protocol II in HR patients aged ≥10 y: 10 mg/m2 days 1 to 7 and 15 to 21.
Patients with initial CNS involvement received additional intrathecal therapy on day 8 and 22 during induction protocol IA.
Cranial radiotherapy was administered after the first reinduction phase (during the interim phase) at the following dose: age 1 to 2 y, 12 Gy (preventive) or 18 Gy (therapeutic for CNS involvement at diagnosis); age ≥2y, 18 Gy (preventive) or 24 Gy (therapeutic for CNS involvement at diagnosis).
Doses were adjusted to WBC count (target range, 2000-3000 per µL).
MRD analysis and stratification
The logistics of the study, cell sample isolation, and identification of the markers for MRD evaluation have been reported elsewhere.1 MRD polymerase chain reaction targets were tested for specificity and sensitivity with the aim of selecting for each patient 2 targets with a sensitivity of at least 10−4 and a quantitative range of at least 10−4 for 1 target and at least 5 × 10−4 for the second target. MRD was measured at 2 time points (TP): day 33 (TP1, at the end of induction IA) and day 78 (TP2, at the end of consolidation IB). Patients were defined as MRD standard risk (MRD-SR) if MRD was found to be negative at both TP1 and TP2, MRD intermediate risk (MRD-IR) when MRD was positive at one or both TPs but at a level of <5 × 10−4 at TP2, or MRD high risk (MRD-HR) if MRD was ≥5 × 10−4 at TP2.2,3
Treatment protocol
All patients were treated according to the AIEOP-BFM ALL 2000 study2,3 in the HR protocol, as described in Table 1. Briefly, in the induction and consolidation phase, all patients underwent 7 days of prephase with steroid therapy (prednisone) and 1 intrathecal dose of MTX, followed by induction protocol IA and consolidation protocol IB; from day 8, patients were randomized to continue steroid treatment with either prednisone (60 mg/m2 per day) or dexamethasone (10 mg/m2 per day) until day 28 and subsequent tapering doses. In the intensification and reinduction phases, patients were randomized to receive either 3 blocks of non–cross-resistant drugs followed by protocol III given 3 times or protocol II given twice. For maintenance therapy, daily 6-mercaptopurine plus weekly MTX was given for a total duration of 24 months from diagnosis. For central nervous system (CNS) directed therapy, intrathecal MTX was given during each treatment phase preceding cranial radiotherapy (dosage by age; see Table 1). Randomized questions were powered to be answered jointly by AIEOP and BFM and are not the focus of this paper.
Indications for allogeneic HSCT from a matched family donor (MFD) or matched unrelated donor (MUD) are shown in Table 2. Although not indicated, some patients were given allogeneic HSCT from an HLA-mismatched donor (MMD). By protocol, HSCT was planned after the third HR block; preparative regimens were according to center strategy and usually included total body irradiation.
Indications for allogeneic HSCT by subgroup
| Subgroup . | Indications for allogeneic HSCT . | Type of donor . | |
|---|---|---|---|
| MFD . | MUD . | ||
| 1 | PPR and: WBC count ≥100 000/mm3 or T-cell ALL or pro–B-cell ALL or MRD ≥10−2 at TP1* | Yes | No |
| 2 | MRD-HR 10−3 at TP2* or t(4;11) and PGR | Yes | No |
| 3 | MRD-HR ≥10−2 at TP2* or no remission at +33 or t(4;11) and PPR | Yes | Yes |
| Subgroup . | Indications for allogeneic HSCT . | Type of donor . | |
|---|---|---|---|
| MFD . | MUD . | ||
| 1 | PPR and: WBC count ≥100 000/mm3 or T-cell ALL or pro–B-cell ALL or MRD ≥10−2 at TP1* | Yes | No |
| 2 | MRD-HR 10−3 at TP2* or t(4;11) and PGR | Yes | No |
| 3 | MRD-HR ≥10−2 at TP2* or no remission at +33 or t(4;11) and PPR | Yes | Yes |
PGR, prednisone good response.
MRD level 10−3 indicates the interval of values between ≥5 × 10−4 and <5 × 10−3; MRD level ≥10−2 includes all values ≥5 × 10−3.
Statistical analysis
The principal end points in the analysis of treatment results were EFS, disease-free survival (DFS), and overall survival (OS). EFS was defined as the time from diagnosis to first failure, which was defined as death during induction therapy, resistance, relapse, death in remission, or development of a second malignant neoplasm. DFS was defined as the time elapsing from CR until relapse, death during CR, or development of a second malignant neoplasm. OS was defined as the time from diagnosis (or time from CR, when stated) to death from any cause. Observation of patients was censored at time of last contact, when no events were observed.
The Kaplan-Meier method was used to estimate the probabilities of EFS, DFS, and OS, with standard errors (SE) calculated according to Greenwood’s formula. Curves were compared using the log-rank test. Kaplan-Meier plots that compared HSCT with chemotherapy were adjusted to account for the waiting time to transplantation. For DFS, the curves originate at a landmark (median time from first CR to transplantation) where the sets at risk do not include patients who had events or whose data were censored before that time; the curves account for patients who underwent HSCT after the landmark by delayed entry. The corresponding analysis of survival is done starting from the same sets at risk at the same landmark as in DFS curves. To deal with lack of proportional hazards between the 2 treatment groups, univariate comparison between these curves was performed at a predefined time point of 5 years from remission based on log-log transformation.12
In Cox regression analyses, treatment was considered to be a time-dependent variable. Thus, each patient was included in the chemotherapy group until transplantation. The time dependence of the treatment effect (ie, nonproportional hazards) was accommodated by including a term for the interaction of time (log transformed) and treatment in the regression analysis.13,14 Estimated hazard ratios in time were reported with 99% confidence interval (CI).
Follow up was updated in June 2013. Analyses were carried out using SAS version 9.2.
The study was registered at the US National Institutes of Health website (http://www.clinicaltrials.gov) as “Combination Chemotherapy based on Risk of Relapse in Treating Young Patients with Acute Lymphoblastic Leukaemia” with the protocol identification number NCT00613457.
Results
Clinical and biological characteristics of the 312 patients in the HR group are reported in Table 3. As shown in column 5, approximately two-thirds of the patients were male; one-third had either age ≥10 years or hyperleukocytosis (≥100 000 white blood cells [WBCs] per μL) or T-cell immunophenotype. Few HR patients presented with known favorable features, namely TEL/AML1 translocation (4.2%) or high hyperdiploidy (DNA index ≥1.16 and <1.6; 10.5%).
Clinical-biological features and related outcome (EFS [SE] estimated at 5 years from diagnosis) in 312 Philadelphia-negative ALL patients according to HR criteria, in hierarchical order
| . | HR criteria in hierarchical order . | 5-y EFS (SE) . | P . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) MRD-HR . | (2) Resistant IA . | (3) t(4;11) . | (4) PPR . | (5) Total . | ||||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |||
| Total | 132 | 42.3 | 17 | 5.4 | 11 | 3.5 | 152 | 48.7 | 312 | 58.9% (2.8)* | ||
| Gender | .03 | |||||||||||
| Male | 85 | 64.4 | 6 | 35.3 | 6 | 54.6 | 102 | 67.1 | 199 | 63.8 | 54.5% (3.6) | |
| Female | 47 | 35.6 | 11 | 64.7 | 5 | 45.4 | 50 | 32.9 | 113 | 36.2 | 66.8% (4.5) | |
| Age | <.001 | |||||||||||
| 1-5 y | 56 | 42.4 | 9 | 52.9 | 3 | 27.3 | 95 | 62.5 | 163 | 52.2 | 66.4% (3.7) | |
| 6-9 y | 31 | 23.5 | 3 | 17.7 | 2 | 18.2 | 27 | 17.8 | 63 | 20.2 | 58.7% (6.2) | |
| 10-17 y | 45 | 34.1 | 5 | 29.4 | 6 | 54.5 | 30 | 19.7 | 86 | 27.6 | 45.1% (5.4) | |
| WBC count (per μL)† | .19 | |||||||||||
| <20 000 | 55 | 41.7 | 9 | 52.9 | 0 | 39 | 25.8 | 103 | 33.1 | 59.4% (4.9) | ||
| ≥20 000 to <100 000 | 39 | 29.5 | 5 | 29.4 | 3 | 27.3 | 66 | 43.7 | 113 | 36.3 | 62.5% (4.6) | |
| ≥100 000 | 38 | 28.8 | 3 | 17.7 | 8 | 72.7 | 46 | 30.5 | 95 | 30.6 | 53.6% (5.1) | |
| Immunophenotype | .008 | |||||||||||
| BCP | 86 | 65.2 | 4 | 23.5 | 11 | 100.0 | 106 | 69.7 | 207 | 66.4 | 63.9% (3.4) | |
| T-cell | 46 | 34.8 | 13 | 76.5 | 0 | 46 | 30.3 | 105 | 33.6 | 49.3% (4.9) | ||
| Translocation | ||||||||||||
| t(4;11) | 5 | 3.8 | 0 | 11 | 100.0 | 0 | 16 | 5.1 | 43.8% (12.4) | |||
| TEL/AML1 | 4 | 3.0 | 0 | 0 | 9 | 5.9 | 13 | 4.2 | 69.2% (12.8) | |||
| DNA index† | .03 | |||||||||||
| ≥1.16 and <1.6 | 7 | 5.7 | 1 | 6.3 | 0 | 23 | 15.8 | 31 | 10.5 | 79.3% (7.6) | ||
| <1.16 or ≥1.6 | 116 | 94.3 | 15 | 93.7 | 11 | 100.0 | 123 | 84.2 | 265 | 89.5 | 57.7% (3.0) | |
| Response to PDN† | .005 | |||||||||||
| PGR | 87 | 66.4 | 8 | 50.0 | 8 | 72.7 | 0 | 103 | 33.2 | 48.1% (5.0) | ||
| PPR | 44 | 33.6 | 8 | 50.0 | 3 | 27.3 | 152 | 100.0 | 207 | 66.8 | 64.9% (3.3) | |
| Resistant to IA‡ | .006 | |||||||||||
| Yes | 15 | 11.4 | 17 | 100.0 | 0 | 0 | 32 | 10.3 | 40.6% (8.7) | |||
| No | 117 | 88.6 | 0 | 11 | 100.0 | 150 | 100.0 | 278 | 89.7 | 61.2% (2.9) | ||
| MRD classification† | <.001 | |||||||||||
| SR | 0 | 1 | 20.0 | 0 | 23 | 19.7 | 24 | 9.2 | 100.0%‡ | |||
| IR | 0 | 4 | 80.0 | 5 | 100.0 | 94 | 80.3 | 103 | 39.8 | 66.9% (4.7) | ||
| HR | 132 | 100.0 | 0 | 0 | 0 | 132 | 51.0 | 45.9% (4.4) | ||||
| . | HR criteria in hierarchical order . | 5-y EFS (SE) . | P . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) MRD-HR . | (2) Resistant IA . | (3) t(4;11) . | (4) PPR . | (5) Total . | ||||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |||
| Total | 132 | 42.3 | 17 | 5.4 | 11 | 3.5 | 152 | 48.7 | 312 | 58.9% (2.8)* | ||
| Gender | .03 | |||||||||||
| Male | 85 | 64.4 | 6 | 35.3 | 6 | 54.6 | 102 | 67.1 | 199 | 63.8 | 54.5% (3.6) | |
| Female | 47 | 35.6 | 11 | 64.7 | 5 | 45.4 | 50 | 32.9 | 113 | 36.2 | 66.8% (4.5) | |
| Age | <.001 | |||||||||||
| 1-5 y | 56 | 42.4 | 9 | 52.9 | 3 | 27.3 | 95 | 62.5 | 163 | 52.2 | 66.4% (3.7) | |
| 6-9 y | 31 | 23.5 | 3 | 17.7 | 2 | 18.2 | 27 | 17.8 | 63 | 20.2 | 58.7% (6.2) | |
| 10-17 y | 45 | 34.1 | 5 | 29.4 | 6 | 54.5 | 30 | 19.7 | 86 | 27.6 | 45.1% (5.4) | |
| WBC count (per μL)† | .19 | |||||||||||
| <20 000 | 55 | 41.7 | 9 | 52.9 | 0 | 39 | 25.8 | 103 | 33.1 | 59.4% (4.9) | ||
| ≥20 000 to <100 000 | 39 | 29.5 | 5 | 29.4 | 3 | 27.3 | 66 | 43.7 | 113 | 36.3 | 62.5% (4.6) | |
| ≥100 000 | 38 | 28.8 | 3 | 17.7 | 8 | 72.7 | 46 | 30.5 | 95 | 30.6 | 53.6% (5.1) | |
| Immunophenotype | .008 | |||||||||||
| BCP | 86 | 65.2 | 4 | 23.5 | 11 | 100.0 | 106 | 69.7 | 207 | 66.4 | 63.9% (3.4) | |
| T-cell | 46 | 34.8 | 13 | 76.5 | 0 | 46 | 30.3 | 105 | 33.6 | 49.3% (4.9) | ||
| Translocation | ||||||||||||
| t(4;11) | 5 | 3.8 | 0 | 11 | 100.0 | 0 | 16 | 5.1 | 43.8% (12.4) | |||
| TEL/AML1 | 4 | 3.0 | 0 | 0 | 9 | 5.9 | 13 | 4.2 | 69.2% (12.8) | |||
| DNA index† | .03 | |||||||||||
| ≥1.16 and <1.6 | 7 | 5.7 | 1 | 6.3 | 0 | 23 | 15.8 | 31 | 10.5 | 79.3% (7.6) | ||
| <1.16 or ≥1.6 | 116 | 94.3 | 15 | 93.7 | 11 | 100.0 | 123 | 84.2 | 265 | 89.5 | 57.7% (3.0) | |
| Response to PDN† | .005 | |||||||||||
| PGR | 87 | 66.4 | 8 | 50.0 | 8 | 72.7 | 0 | 103 | 33.2 | 48.1% (5.0) | ||
| PPR | 44 | 33.6 | 8 | 50.0 | 3 | 27.3 | 152 | 100.0 | 207 | 66.8 | 64.9% (3.3) | |
| Resistant to IA‡ | .006 | |||||||||||
| Yes | 15 | 11.4 | 17 | 100.0 | 0 | 0 | 32 | 10.3 | 40.6% (8.7) | |||
| No | 117 | 88.6 | 0 | 11 | 100.0 | 150 | 100.0 | 278 | 89.7 | 61.2% (2.9) | ||
| MRD classification† | <.001 | |||||||||||
| SR | 0 | 1 | 20.0 | 0 | 23 | 19.7 | 24 | 9.2 | 100.0%‡ | |||
| IR | 0 | 4 | 80.0 | 5 | 100.0 | 94 | 80.3 | 103 | 39.8 | 66.9% (4.7) | ||
| HR | 132 | 100.0 | 0 | 0 | 0 | 132 | 51.0 | 45.9% (4.4) | ||||
BCP, B-cell precursor; IR, intermediate risk; PDN, prednisone; SR, standard risk.
The 5-year EFS (SE) in the non-HR patients (n = 1687) treated in Italy in AIEOP-BFM ALL 2000 was 83.0% (0.9), with a survival of 93.0% (0.6).
A relapse occurred at 6.3 years from diagnosis.
WBC: 1 not known; DNA index: 16 not known; Response to PDN: 2 not known; Resistant to IA: 2 not applicable; MRD classification: 53 not known.
Criteria for inclusion of children in the HR group are presented in Table 3, in hierarchical order from column 1 to column 4. Thus, in column 1, the subset of 132 patients (42.3%) includes those who presented with MRD ≥ 5 × 10−4 at day 78, regardless of the remaining features, and so on for the subsequent columns 2 to 4. Consequently, column 4 identifies 152 (48.7%) patients who were defined as HR only because of their poor response to prednisone. Only 17 (5.4%) and 11 (3.5%) patients were hierarchically classified at HR for no CR at day +33 or t(4;11) translocation, respectively (columns 2 and 3). Supplemental Table 1 (available on the Blood Web site) shows in detail those patients who presented a combination of HR criteria.
Median follow-up was 8.9 years from diagnosis, with a minimum potential follow-up of 7 years and with most patients updated in the last 2 years (180 of 209 alive). The overall 5-year and 10-year EFS was 58.9% (SE = 2.8) and 55.2% (SE = 3.0) with a corresponding survival of 68.9% (SE = 2.6) and 66.8% (SE = 2.7), respectively (Figure 1). The univariate description of EFS by clinical features shows trends in keeping with the role of conventional prognostic factors (gender, age, WBC count, and immunophenotype) (Table 3). Among the 13 TEL/AML1-positive patients, 4 relapses were recorded: 2 in the 4 children who had MRD-HR and 2 in the 9 at HR only because of PPR. The 31 high-hyperdiploid patients had a 5-year EFS of 79.3% (SE = 7.6), as compared with 57.7% (SE = 3.0) in patients with a DNA index <1.16 or ≥1.6. Interestingly, 24 HR patients were MRD negative at both time points (MRD-SR) and only one of them relapsed at 6.3 years after the diagnosis. Table 4 presents outcome by subgroups (in hierarchical order, columns 1-4) and overall (column 5). One PPR patient died during phase IA of sepsis. Thirty-two patients were not in CR at the end of induction phase IA (15 of them were in the MRD-HR subgroup); out of the 26 who eventually achieved CR, 13 were alive in continuous CR at 5 years. Only 6 were eventually resistant to therapy, because they did not achieve CR by the end of the chemotherapy blocks (1.9% of the HR patients and 0.3% of the whole AIEOP study population); all of them subsequently died.
Estimates of EFS and survival in 312 children with ALL classified as HR in the AIEOP-BFM 2000 protocol and treated in AIEOP centers.
Estimates of EFS and survival in 312 children with ALL classified as HR in the AIEOP-BFM 2000 protocol and treated in AIEOP centers.
Distribution of events in 312 Philadelphia-negative ALL patients according to HR criteria, in hierarchical order
| . | HR criteria (in hierarchical order) . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) MRD-HR . | (2) Resistant IA . | (3) t(4;11) . | (4) PPR . | (5) Total . | ||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| Total | 132 | 42.3 | 17 | 5.4 | 11 | 3.5 | 152 | 48.7 | 312 | |
| Death in induction | 0 | 0 | 0 | 1 | 0.7 | 1 | 0.3 | |||
| Resistant to protocol | 3 | 2.3 | 3 | 17.6 | 0 | 0 | 6 | 1.9 | ||
| Relapses (deaths) | 56 (46) | 42.4 | 6 (6) | 35.3 | 5 (4) | 45.4 | 38 (20) | 25.0 | 105 (76) | 33.7 |
| Timing | ||||||||||
| <6 mo | 7 | 5.3 | 0 | 0 | 1 | 0.7 | 8 | 2.5 | ||
| 6-18 mo | 22 | 16.6 | 2 | 11.8 | 5 | 45.4 | 14 | 9.2 | 43 | 13.8 |
| 18-30 mo | 19 | 14.4 | 3 | 17.6 | 0 | 12 | 7.9 | 34 | 10.9 | |
| ≥30 mo | 8 | 6.1 | 1 | 5.9 | 0 | 11 | 7.2 | 20 | 6.5 | |
| Site | ||||||||||
| BM | 37 | 28.0 | 4 | 23.5 | 4 | 36.3 | 22 | 14.5 | 67 | 21.5 |
| CNS | 5 | 3.8 | 0 | 0 | 4 | 2.6 | 9 | 2.9 | ||
| Testis | 3 | 2.3 | 0 | 1 | 9.1 | 1 | 0.7 | 5 | 1.6 | |
| BM + others | 8 | 6.0 | 1 | 5.9 | 0 | 7 | 4.6 | 16 | 5.1 | |
| Others | 3 | 2.3 | 1 | 5.9 | 0 | 4 | 2.6 | 8 | 2.6 | |
| Second malignant neoplasm | 5* | 3.8 | 0 | 0 | 3† | 2.0 | 8 | 2.6 | ||
| Death in CCR | 10 | 7.6 | 1 | 5.9 | 2 | 18.2 | 2 | 1.3 | 15 | 4.8 |
| After chemotherapy‡ | 2 | 1.5 | 1 | 5.9 | 1 | 9.1 | 2 | 1.3 | 6 | 1.9 |
| After HSCT | 8 | 6.1 | 0 | 1 | 9.1 | 0 | 9 | 2.9 | ||
| CCR | 58 | 43.9 | 7 | 41.2 | 4 | 36.4 | 108 | 71.0 | 177 | 56.7 |
| 5-y EFS (SE) | 45.9% (4.4) | 41.2% (11.9) | 36.4% (14.5) | 74.0% (3.6) | 58.9% (2.8) | |||||
| 10-y EFS (SE) | 42.9% (4.4) | 41.2% (11.9) | 36.4% (14.5) | 68.8% (4.1) | 55.2% (3.0) | |||||
| P < .001 | ||||||||||
| 5-y survival (SE) | 54.1% (4.4) | 41.2% (11.9) | 45.5% (15.0) | 86.7% (2.8) | 68.9% (2.6) | |||||
| 10-y survival (SE) | 52.5% (4.4) | 41.2% (11.9) | 45.5% (15.0) | 83.8% (3.0) | 66.8% (2.7) | |||||
| P < .001 | ||||||||||
| . | HR criteria (in hierarchical order) . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) MRD-HR . | (2) Resistant IA . | (3) t(4;11) . | (4) PPR . | (5) Total . | ||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| Total | 132 | 42.3 | 17 | 5.4 | 11 | 3.5 | 152 | 48.7 | 312 | |
| Death in induction | 0 | 0 | 0 | 1 | 0.7 | 1 | 0.3 | |||
| Resistant to protocol | 3 | 2.3 | 3 | 17.6 | 0 | 0 | 6 | 1.9 | ||
| Relapses (deaths) | 56 (46) | 42.4 | 6 (6) | 35.3 | 5 (4) | 45.4 | 38 (20) | 25.0 | 105 (76) | 33.7 |
| Timing | ||||||||||
| <6 mo | 7 | 5.3 | 0 | 0 | 1 | 0.7 | 8 | 2.5 | ||
| 6-18 mo | 22 | 16.6 | 2 | 11.8 | 5 | 45.4 | 14 | 9.2 | 43 | 13.8 |
| 18-30 mo | 19 | 14.4 | 3 | 17.6 | 0 | 12 | 7.9 | 34 | 10.9 | |
| ≥30 mo | 8 | 6.1 | 1 | 5.9 | 0 | 11 | 7.2 | 20 | 6.5 | |
| Site | ||||||||||
| BM | 37 | 28.0 | 4 | 23.5 | 4 | 36.3 | 22 | 14.5 | 67 | 21.5 |
| CNS | 5 | 3.8 | 0 | 0 | 4 | 2.6 | 9 | 2.9 | ||
| Testis | 3 | 2.3 | 0 | 1 | 9.1 | 1 | 0.7 | 5 | 1.6 | |
| BM + others | 8 | 6.0 | 1 | 5.9 | 0 | 7 | 4.6 | 16 | 5.1 | |
| Others | 3 | 2.3 | 1 | 5.9 | 0 | 4 | 2.6 | 8 | 2.6 | |
| Second malignant neoplasm | 5* | 3.8 | 0 | 0 | 3† | 2.0 | 8 | 2.6 | ||
| Death in CCR | 10 | 7.6 | 1 | 5.9 | 2 | 18.2 | 2 | 1.3 | 15 | 4.8 |
| After chemotherapy‡ | 2 | 1.5 | 1 | 5.9 | 1 | 9.1 | 2 | 1.3 | 6 | 1.9 |
| After HSCT | 8 | 6.1 | 0 | 1 | 9.1 | 0 | 9 | 2.9 | ||
| CCR | 58 | 43.9 | 7 | 41.2 | 4 | 36.4 | 108 | 71.0 | 177 | 56.7 |
| 5-y EFS (SE) | 45.9% (4.4) | 41.2% (11.9) | 36.4% (14.5) | 74.0% (3.6) | 58.9% (2.8) | |||||
| 10-y EFS (SE) | 42.9% (4.4) | 41.2% (11.9) | 36.4% (14.5) | 68.8% (4.1) | 55.2% (3.0) | |||||
| P < .001 | ||||||||||
| 5-y survival (SE) | 54.1% (4.4) | 41.2% (11.9) | 45.5% (15.0) | 86.7% (2.8) | 68.9% (2.6) | |||||
| 10-y survival (SE) | 52.5% (4.4) | 41.2% (11.9) | 45.5% (15.0) | 83.8% (3.0) | 66.8% (2.7) | |||||
| P < .001 | ||||||||||
Two AML (0.6, 2.7 y from diagnosis), 1 MDS (1.6), 1 Ewing sarcoma (3.7), and 1 malignant reticuloendotheliosis (1.2).
Two glioblastomas (4.2 and 4.8 y from diagnosis) and 1 AML (1.7).
Cause of death and time of death were hemolytic uremic syndrome (n = 1, at 0.5 y from diagnosis), sepsis (n = 2, at 0.2 and 0.3 y), pneumonia (n = 2, at 0.5 and 1.3 y), and brain hemorrhage (n = 1, at 1.0 y).
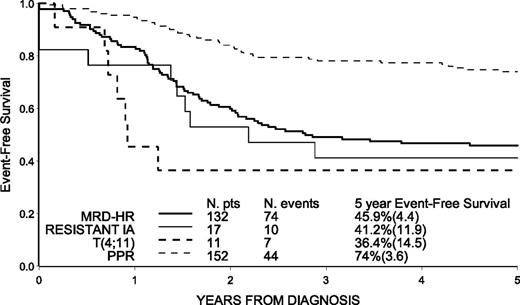
Relapse was diagnosed in 105 patients and it occurred late (≥30 months from diagnosis) only in 20 patients (6 in extramedullary sites). BM was involved in most relapses (n = 83; 79%). A total of 8 second malignant neoplasms were reported, all in nontransplanted patients (described in Table 4), of whom 2 glioblastomas could be related to cranial radiotherapy. Death in continuous CR (CCR) occurred in 15 patients (of whom 9 died after HSCT). The EFS by HR criteria shows that outcome is relatively favorable in patients at HR only because of PPR (5-year EFS of 74.0%; SE = 3.6) whereas it is markedly worse, in the order of 40%, in the remaining patients, mostly represented by the large MRD-HR subgroup (P < .001; Figure 2).
Estimates of EFS in 312 children with HR-ALL by HR criteria (hierarchical order).
Estimates of EFS in 312 children with HR-ALL by HR criteria (hierarchical order).
Results of HSCT
A total of 226 patients who satisfied criteria for HSCT in first remission (see Table 2) had a 5-year DFS and OS of 52.9% (SE = 3.3) and 63.1% (SE = 3.2), respectively. Overall, 81 patients were transplanted at a median of 5.7 months after achieving CR1 from an MFD (47 patients; median time to transplantation, 5.3 months), MUD (19 patients; median time to transplantation, 6.4 months), or MMD (15 patients; median time to transplantation, 7.0 months). Results by type of donor were similar, with a 5-year DFS from HSCT of 55.2% (SE = 7.3), 63.2% (SE = 11.1), and 53.3% (SE = 12.8) after MFD, MUD, and MMD HSCT, respectively (P = .86; supplemental Table 2).
Comparison of outcome after HSCT or chemotherapy has been performed adjusting by the overall median waiting time to transplant within the 3 subgroups indicated in Table 2 by eligibility criteria to HSCT. In the subgroup of 72 patients who were at HR only for PPR and were eligible for MFD HSCT due to hyperleukocytosis or T-cell immunophenotype (Table 2), with only 12 transplanted patients (as expected by the probability of having an MFD), the overall 5-year DFS was 66.6% (SE = 5.6) and survival was 81.9% (SE = 4.5). As shown in Table 5, the relapse rate was 16.7% after HSCT and 38.3% after chemotherapy, with CCR rates (not adjusted for waiting time to HSCT) of 83.3% and 58.3%, respectively. The adjusted 5-year DFS was 83.3% (SE = 10.8) and 67.7% (SE = 6.3), respectively, for transplanted or chemotherapy patients (P = .31), with a survival of 83.3% (SE = 10.8) and 81.9% (SE = 5.4) (P = .91; Figure 3A-B). Because some relapses in the chemotherapy patients occurred late, OS might change with a longer follow-up.
Outcome of HR-ALL children eligible to HSCT in first remission and treated with HSCT or chemotherapy in 3 subgroups
| . | Subgroup 1* (72 patients) . | Subgroup 2* (92 patients) . | Subgroup 3* (62 patients) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy . | HSCT . | Chemotherapy . | HSCT . | Chemotherapy . | HSCT . | |||||||
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | |
| Total | 60 | 12 | 64 | 28 | 21 | 41 | ||||||
| Relapses (deaths) | 23 (12) | 38.3 | 2 (2) | 16.7 | 29 (25) | 45.3 | 9 (7) | 32.1 | 14 (12) | 66.7 | 15 (12) | 36.6 |
| Time from first CR | ||||||||||||
| <6 mo | 3 | 0 | 4 | 0 | 6 | 0 | ||||||
| 6-18 mo | 8 | 2 | 8 | 8 | 4 | 11 | ||||||
| 18-30 mo | 6 | 0 | 15 | 0 | 1 | 2 | ||||||
| ≥30 mo | 6 | 0 | 2 | 1 | 3 | 2 | ||||||
| Site | ||||||||||||
| BM | 11 | 1 | 17 | 7 | 10 | 11 | ||||||
| CNS | 4 | 0 | 4 | 0 | 1 | 0 | ||||||
| Testis | 0 | 0 | 2 | 2 | 0 | 0 | ||||||
| BM + other | 5 | 1 | 6 | 0 | 2 | 1 | ||||||
| Other | 3 | 0 | 0 | 0 | 1 | 3 | ||||||
| SMN | 1 | 1.7 | 0 | 5 | 7.8 | 0 | 0 | 0 | ||||
| Death in CCR | 1 | 1.7 | 0 | 2 | 3.1 | 4 | 14.3 | 2 | 9.5 | 5 | 12.2 | |
| CCR | 35 | 58.3 | 10 | 83.3 | 28 | 43.8 | 15 | 53.6 | 5 | 23.8 | 21 | 51.2 |
| . | Subgroup 1* (72 patients) . | Subgroup 2* (92 patients) . | Subgroup 3* (62 patients) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy . | HSCT . | Chemotherapy . | HSCT . | Chemotherapy . | HSCT . | |||||||
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | |
| Total | 60 | 12 | 64 | 28 | 21 | 41 | ||||||
| Relapses (deaths) | 23 (12) | 38.3 | 2 (2) | 16.7 | 29 (25) | 45.3 | 9 (7) | 32.1 | 14 (12) | 66.7 | 15 (12) | 36.6 |
| Time from first CR | ||||||||||||
| <6 mo | 3 | 0 | 4 | 0 | 6 | 0 | ||||||
| 6-18 mo | 8 | 2 | 8 | 8 | 4 | 11 | ||||||
| 18-30 mo | 6 | 0 | 15 | 0 | 1 | 2 | ||||||
| ≥30 mo | 6 | 0 | 2 | 1 | 3 | 2 | ||||||
| Site | ||||||||||||
| BM | 11 | 1 | 17 | 7 | 10 | 11 | ||||||
| CNS | 4 | 0 | 4 | 0 | 1 | 0 | ||||||
| Testis | 0 | 0 | 2 | 2 | 0 | 0 | ||||||
| BM + other | 5 | 1 | 6 | 0 | 2 | 1 | ||||||
| Other | 3 | 0 | 0 | 0 | 1 | 3 | ||||||
| SMN | 1 | 1.7 | 0 | 5 | 7.8 | 0 | 0 | 0 | ||||
| Death in CCR | 1 | 1.7 | 0 | 2 | 3.1 | 4 | 14.3 | 2 | 9.5 | 5 | 12.2 | |
| CCR | 35 | 58.3 | 10 | 83.3 | 28 | 43.8 | 15 | 53.6 | 5 | 23.8 | 21 | 51.2 |
SMN, second malignant neoplasm.
See Table 2 for descriptions of subgroups.
Outcome of HR-ALL children eligible for HSCT in first remission and treated with HSCT or chemotherapy only. Patients are divided in 3 subgroups by transplant indications (see also Table 2): 72 PPR patients eligible for HSCT because of ≥100 000 WBC or T-cell or pro–B-cell immunophenotype or MRD ≥10−2 at TP1, of whom 12 underwent HSCT (panels A and B for DFS and survival, respectively); 92 patients eligible for HSCT because of MRD level 10−3 at TP2 or t(4;11) and prednisone good response of whom 28 underwent HSCT (panels C and D for DFS and survival); and 62 patients eligible for HSCT because of MRD level ≥10−2 at TP2 or no CR after induction IA or t(4;11) and prednisone poor response, of whom 41 underwent HSCT (panels E and F for DFS and survival). The curves were adjusted for waiting time to transplantation, so that the 0 on the time axis corresponds to the median time from first CR to HSCT (5.7 months); patients were assigned to the treatment group in a time-dependent fashion. Patients with relapses or deaths occurring before the median waiting time to transplant thus are not counted. HSCT, hematopoietic stem cell transplantation.
Outcome of HR-ALL children eligible for HSCT in first remission and treated with HSCT or chemotherapy only. Patients are divided in 3 subgroups by transplant indications (see also Table 2): 72 PPR patients eligible for HSCT because of ≥100 000 WBC or T-cell or pro–B-cell immunophenotype or MRD ≥10−2 at TP1, of whom 12 underwent HSCT (panels A and B for DFS and survival, respectively); 92 patients eligible for HSCT because of MRD level 10−3 at TP2 or t(4;11) and prednisone good response of whom 28 underwent HSCT (panels C and D for DFS and survival); and 62 patients eligible for HSCT because of MRD level ≥10−2 at TP2 or no CR after induction IA or t(4;11) and prednisone poor response, of whom 41 underwent HSCT (panels E and F for DFS and survival). The curves were adjusted for waiting time to transplantation, so that the 0 on the time axis corresponds to the median time from first CR to HSCT (5.7 months); patients were assigned to the treatment group in a time-dependent fashion. Patients with relapses or deaths occurring before the median waiting time to transplant thus are not counted. HSCT, hematopoietic stem cell transplantation.
A total of 92 patients were eligible for MFD HSCT due to t(4;11) translocation only or an MRD level of 10−3 at TP2 (Table 2, subgroup 2), and 28 of them were transplanted. The overall 5-year DFS and survival was 46.1% (SE = 5.3) and 56.9% (SE = 5.2). The relapse rate was 32.1% after HSCT and 45.3% after chemotherapy, with CCR rates (not adjusted for waiting time to HSCT) of 53.6% and 43.8%, respectively (Table 5). The adjusted 5-year DFS was 51.1% (SE = 9.6) and 47.2% (SE = 6.6) (P = .74) and survival was 59.3% (SE = 9.4) and 59.3% (SE = 6.6) (P = .99) for HSCT and chemotherapy patients, respectively (Figure 3C-D). The Cox regression model was applied in this relatively large subgroup in order to describe the effect of HSCT on DFS and survival compared with chemotherapy (Table 6). The early disadvantage estimated for HSCT patients in the first year from first CR, both for DFS and survival, was counterbalanced by a progressively lower hazard of events in subsequent years. According to the Cox model on DFS, the hazard of failure (relapse or death in remission) was reduced, although not significantly (ie, the CI always includes the value 1 for the hazard ratio), to one-third at 3 years (hazard ratio = 0.30; 99% CI, 0.06-1.52) in HSCT patients as compared with those given chemotherapy only. The same pattern was observed for the Cox model on survival.
Cox regression model of the effect of HSCT vs chemotherapy on DFS and survival
| Time from first CR* . | Subgroup 2 . | Subgroup 3 . |
|---|---|---|
| HSCT vs chemotherapy on DFS | ||
| At 1 y | 1.54 (0.59-4.03) | 0.89 (0.32-2.46) |
| At 2 y | 0.54 (0.17-1.71) | 0.51 (0.15-1.73) |
| At 3 y | 0.30 (0.06-1.52) | 0.37 (0.08-1.70) |
| At 4 y | 0.19 (0.03-1.48) | 0.29 (0.05-1.74) |
| At 5 y | 0.14 (0.01-1.46) | 0.25 (0.03-1.80) |
| HSCT vs chemotherapy on survival | ||
| At 1 y | 1.27 (0.33-4.95) | 0.53 (0.18-1.60) |
| At 2 y | 0.78 (0.31-1.99) | 0.42 (0.12-1.47) |
| At 3 y | 0.59 (0.18-1.95) | 0.37 (0.07-2.04) |
| At 4 y | 0.48 (0.10-2.22) | 0.34 (0.04-2.74) |
| At 5 y | 0.41 (0.07-2.56) | 0.31 (0.03-3.50) |
| Time from first CR* . | Subgroup 2 . | Subgroup 3 . |
|---|---|---|
| HSCT vs chemotherapy on DFS | ||
| At 1 y | 1.54 (0.59-4.03) | 0.89 (0.32-2.46) |
| At 2 y | 0.54 (0.17-1.71) | 0.51 (0.15-1.73) |
| At 3 y | 0.30 (0.06-1.52) | 0.37 (0.08-1.70) |
| At 4 y | 0.19 (0.03-1.48) | 0.29 (0.05-1.74) |
| At 5 y | 0.14 (0.01-1.46) | 0.25 (0.03-1.80) |
| HSCT vs chemotherapy on survival | ||
| At 1 y | 1.27 (0.33-4.95) | 0.53 (0.18-1.60) |
| At 2 y | 0.78 (0.31-1.99) | 0.42 (0.12-1.47) |
| At 3 y | 0.59 (0.18-1.95) | 0.37 (0.07-2.04) |
| At 4 y | 0.48 (0.10-2.22) | 0.34 (0.04-2.74) |
| At 5 y | 0.41 (0.07-2.56) | 0.31 (0.03-3.50) |
Data are presented as hazard ratio (99% CI).
The Cox model was not applied to subgroup 1 due to the small number of patients transplanted.
Finally, the third subgroup of patients (n = 62) was also eligible for MUD HSCT owing to MRD ≥ 10−2 at TP2, no remission at day +33, or t(4;11) translocation and PPR (Table 2, subgroup 3) and included 41 transplanted patients. The overall 5-year DFS and survival was 46.8% (SE = 6.3) and 50.0% (SE = 6.4). The relapse rate was 36.6% after HSCT and 66.7% after chemotherapy, with CCR rates (not adjusted for waiting time to HSCT) of 51.2% and 23.8%, respectively (Table 5). The adjusted 5-year DFS was 50.5% (SE = 8.0) vs 54.7% (SE = 13.6) (P = .79) and survival was 58.1% (SE = 8.0) vs 51.7% (SE = 13.4) (P = .68) (Figure 3E-F). The marked difference between curves adjusted or not adjusted is explained by the fact that, in patients treated with chemotherapy, 5 relapses and the 2 deaths in CCR occurred within 5.7 months after the first CR (ie, before the median waiting time to HSCT) and are thus not counted in the adjusted DFS and OS curves. The Cox model does not show a significant difference in time for both DFS and survival.
A further subgroup analysis was performed to assess the impact of HSCT according to immunophenotype in patients belonging to subgroups 2 and 3. As shown in supplemental Figure 1, more favorable, although not statistically significant, results were obtained in T-cell ALL patients given HSCT (n = 26, of whom 20 had MRD-HR) compared with those treated with chemotherapy only (n = 29, of whom 25 had MRD-HR). The 5-year DFS was 59.7% (SE = 9.8) vs 31.9% (SE = 9.8; P = .06) and survival was 59.7% (SE = 9.8) vs 40.4% (SE = 10.2). Conversely, in B-cell precursor ALL, results were slightly more favorable in patients treated with chemotherapy only (5-year DFS = 56.4% [SE = 7.2] vs 45.2% [SE = 7.6]; P = .29).
Discussion
In the AIEOP-BFM ALL 2000 study, about 15% (312 of 1999 patients) of the AIEOP patients with noninfant and non–Philadelphia-positive ALL were included in the HR group.
About one-half of them (n = 152) were at HR only because of PPR. They achieved good results, with a 5-year EFS of 74.0% and an OS of 86.7% (Table 4). This finding confirms the benefit of a comprehensive BFM-backbone therapy intensified by polychemotherapy blocks, as previously reported.8,9 Although intensive, this chemotherapy regimen was relatively safe, being associated with only 2 deaths in CCR. Interestingly, among the 152 patients at HR only for PPR, 23 were MRD-SR and their EFS was 100% at 5 years; it should be noted, however, that a relapse occurred at 6.3 years from diagnosis. These data suggest that future treatment strategies for these patients should focus on limiting long-term toxicity. Study questions for these patients could thus investigate the impact of omitting cranial radiotherapy or reducing cumulative doses of anthracyclines and/or steroids to minimize the risk of secondary malignant brain tumors,15,16 intellectual impairment,17 heart toxicity,18 and osteonecrosis.19,20
Half of the HR patients (n = 160) could be regarded as very HR, because they presented, in a cascade order, with MRD-HR levels (n = 132) or no CR after 5 weeks of therapy (n = 17) or t(4;11) translocation (n = 11), with some of them having a combination of these features. In the studies done in the early 1990s, where MRD was assessed but not used for stratification, MRD-HR was associated with a dismal prognosis (5-year EFS of 20%).4 Results obtained in the AIEOP-ALL 2000 study suggest that the conventional BFM therapy, intensified with polychemotherapy blocks, although of some benefit, remains unsatisfactory for these patients (5-year EFS of 44.7%).
Overall, 32 patients were not in CR after the first 5 weeks of therapy. Of the 12 patients with an M3 marrow, 8 achieved CR subsequently but only 2 remain in CCR (both after HSCT); of the remaining 20 patients (17 with an M2 marrow and 3 with persistent extramedullary disease), 18 obtained a CR subsequently, with 11 remaining in CCR. Thus, a relevant fraction of patients not responding to the initial 5 weeks of therapy may be rescued by subsequent treatment, although prognosis remains dismal for patients with an M3 marrow, as recently reported.21 True resistance to treatment, as defined in our protocol, was exceedingly rare (6 patients, or 0.3% of all patients) and invariably associated with fatal outcome. The last subgroup of very HR patients is identified by the t(4;11) translocation, and their prognosis was poor regardless of MRD response (3 out of 5 MRD-HR and 4 out of 11 non–MRD-HR patients remain in CR). These poor results despite intensive BFM therapy, including HSCT, suggest that these patients need innovative therapies, possibly targeted for specific biological features.22
After describing raw numbers of events, the statistical comparison of HSCT and chemotherapy always accounted for waiting time to transplantation.23 The curves thus originate at the landmark of 5.7 months after first CR and do not show a significant advantage for HSCT over chemotherapy on DFS. The more complex analysis performed with the Cox model on DFS in the larger subgroup of patients (subgroup 2) shows how the initial advantage of chemotherapy changes into a disadvantage in favor of HSCT as time increases, due to late relapses after chemotherapy. The univariate analysis, with comparison of DFS curves at 5 years, indicates that the Cox model may overstate the late-term benefit of HSCT in this subgroup.
Interestingly, we found that T-cell ALL patients belonging to subgroups 2 and 3 seemed to benefit from HSCT both for DFS and survival. This difference is due to the very poor outcome of T-cell ALL patients with HR features when treated with chemotherapy (5-year DFS of 31.9% and survival of 40.4%).
Due to the high impact of MRD criteria for HSCT eligibility, these results are not directly comparable with previous I-BFM-SG experience, which showed an advantage for patients with an MFD24 or T-cell ALL.25 A study contemporary to AIEOP-BFM ALL 2000, which used the same stratification criteria, has been published recently.26 In that study, however, different postinduction/consolidation HR chemotherapy blocks were used and indications for MUD HSCT were broader, resulting in about 50% of HR patients undergoing HSCT (compared with 25% of patients in our study). Crucial for these patients might be the identification of an effective consolidation disease control with MRD level reduction before HSCT. (Unfortunately, these data are not available in our series.)
In conclusion, our data show that current BFM therapy is quite effective for patients at HR only because of PPR but remains inadequate for HR patients identified by high MRD levels (≥5 × 10−4) after induction/consolidation therapy. Furthermore, these results do not support the indication for HSCT in patients at HR only for PPR (who might instead benefit from optimization of therapy aimed at reducing long-term toxicity or sequelae) and indicate that new treatment strategies are needed in patients with high MRD levels, especially those with the T-cell ALL immunophenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the contribution of the referees, who greatly improved the paper.
This work was supported by Comitato M.L. Verga and Fondazione Tettamanti (Monza), Fondazione Città della Speranza, Fondazione Cariparo (Padova), and Associazione Italiana per la Ricerca sul Cancro (ab, mgv IG 5017).
Authorship
Contribution: V.C. and G.M. coordinated the study; M.G.V. was the study statistician; G.B. and A.B. were responsible for diagnosis and polymerase chain reaction MRD analyses; D.S. contributed to study coordination and data revision; V.C., M.G.V., L.L.N., F.L., M.A., G.B., and A.B. wrote the manuscript; and V.C, M.G.V., R.P., M.C.P., F.L., E.B., L.L.N., N.S., M.A., O.Z., A.P., A.M.T., C.M., F.C., M.Z., G.C., P.T., G.L.B., L.D.N., D.S., A.C., C.R., A.B., G.M., G.B. participated in the protocol development, study supervision, and data interpretation of this study and have reviewed and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea Biondi, Department of Pediatrics, Ospedale S. Gerardo, University of Milano-Bicocca, Fondazione MBBM, Via Pergolesi, 33, 20900 Monza (MB), Italy; e-mail: abiondi.unimib@gmail.com.
References
Author notes
V.C. and M.G.V. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal