Key Points
Patients up to age 70 years with CML treated within a decentralized health care setting had a relative survival close to 1.0.
Sokal, but not EUTOS, score at diagnosis predicted overall and relative survival in a population-based cohort of patients with CML.
Abstract
Clinical management guidelines on malignant disorders are generally based on data from clinical trials with selected patient cohorts. In Sweden, more than 95% of all patients diagnosed with chronic myeloid leukemia (CML) are reported to the national CML registry, providing unique possibilities to compile population-based information. This report is based on registry data from 2002 to 2010, when a total of 779 patients (425 men, 354 women; median age, 60 years) were diagnosed with CML (93% chronic, 5% accelerated, and 2% blastic phase) corresponding to an annual incidence of 0.9/100 000. In 2002, approximately half of the patients received a tyrosine kinase inhibitor as initial therapy, a proportion that increased to 94% for younger (<70 years) and 79% for older (>80 years) patients during 2007-2009. With a median follow-up of 61 months, the relative survival at 5 years was close to 1.0 for patients younger than 60 years and 0.9 for those aged 60 to 80 years, but only 0.6 for those older than 80 years. At 12 months, 3% had progressed to accelerated or blastic phase. Sokal, but not European Treatment and Outcome Study, high-risk scores were significantly linked to inferior overall and relative survival. Patients living in university vs nonuniversity catchment areas more often received tyrosine kinase inhibitors up front but showed comparable survival.
Introduction
Clinical management and outcome in chronic myeloid leukemia (CML) have improved dramatically since the introduction of imatinib and other tyrosine kinase inhibitors (TKIs).1-3 Today, only a minority of patients transform to blast crisis and die of their disease. However, improvement in survival in the elderly CML patient population has been more modest, possibly because of an underuse of imatinib and second-generation TKIs.4
Several prognostic scores, based on variables recorded at diagnosis, may aid in identifying patients with a less-favorable outcome with standard treatment.5,6 More recently, the European Treatment and Outcome Study (EUTOS) score, based on spleen size and percentage of basophils in peripheral blood at diagnosis in patients treated within clinical trials and dividing patients into 2 different prognostic groups (ie, low and high risk), was claimed to be superior to the previously published Sokal and Hasford/Euro scores in prognostic ability.7 However, the relevance of these scores in patients with CML receiving treatment outside clinical trials needs further clarification.8,9
In CML, as well as in many other diseases, clinical management guidelines are mainly based on results from clinical trials performed on selected study populations in which elderly patients and those with significant and/or multiple comorbidities are underrepresented.10-12 Typically, clinical trial patients have been treated within a university hospital setting. Thus, collecting results from population-based registries with full coverage of the target population would reduce the effect of selection on outcome and provide important complementary data to those obtained from clinical trials.13 Moreover, communicating reports from such registries in timely manner to all treating clinicians may also be a way to improve the quality of care, including routines for diagnostics and follow-up.13-15 This is particularly important in countries such as Sweden, where the management of patients even with relatively rare malignancies, such as CML, to a large extent is decentralized to smaller, nonuniversity hospitals.
The Swedish CML Registry, containing data obtained at diagnosis and follow-up from all adult (18 years of age or older) Swedish patients with CML diagnosed in 2002 and later, irrespective of treatment, has so far included more than 900 patients.16 Evaluating updated information from this registry, we here present data on incidence, clinical characteristics at diagnosis, clinical outcome, and adherence to national CML management guidelines. In addition, in this population-based CML cohort, we have also validated the Sokal, Hasford/Euro, and EUTOS prognostic scores and their ability to predict survival. Finally, we have investigated whether living in a university hospital catchment area versus outside such an area has any major effect on CML management and survival.
Patients and methods
The Swedish CML registry
Modeled on the previously established Swedish Acute Leukemia Registry,15 the Swedish CML Registry was founded in 2002 by the Swedish CML group and the Swedish Society of Hematology. It is supported by the Swedish Board for Health and Welfare and managed in collaboration with the Regional Cancer Centers (RCCs) in each of the 6 Swedish health care regions, covering in total a population of 9.5 million people. In each region, there are 1 or 2 university hospitals and up to 12 county hospitals treating patients with CML. Clinicians are expected to report all patients with newly diagnosed CML to this registry. Starting in 2007, reports are submitted electronically to the central database. In addition, pathologists and clinicians are obliged by law to report all new cases to the Swedish Cancer Registry.17 Because of this dual reporting system, it is possible for the RCCs to identify and request reports from cases missing in the CML registry.
Inclusion criteria and variables
Swedish inhabitants diagnosed on January 1, 2002, or later with CML in chronic (CP), accelerated (AP), or blastic (BP) phase were included after informed consent. In addition, patients with a clinico-pathological picture typical of CML, but without a cytogenetically or molecularly confirmed diagnosis of CML (karyotyping, fluorescence in situ hybridization [FISH], and/or reverse transcription-polymerase chain reaction [RT-PCR] of BCR/ABL1 not performed or failed), were eligible. However, the latter group (n = 24) was not included in the outcome analyses of this report (see following).
At diagnosis, sex, age, clinical stage (ie, CP, AP, or BP), whether treatment aim was complete cytogenetic response (CCgR)/major molecular response (MMR) or “palliation” (ie, symptomatic treatment with a non-TKI regimen and no hematopoietic stem cell transplantation [HSCT]), inclusion in an up-front clinical trial, and details on cytogenetic/molecular work-up, as well as spleen size (by palpation) and results of basic hematology tests, were registered. At annual follow-up, disease status, response to therapy, and survival were to be reported. From 2007 onward, the registry contains more-detailed data on therapy and outcome, including molecular responses.
This report includes data on 779 patients diagnosed between January 1, 2002, and December 31, 2010. This figure corresponds to 97% of all patients reported by pathologists to the compulsory Swedish Cancer Registry. In addition, results from 12-month follow-up in 589 patients diagnosed between 2002 and 2009 were included. Survival was validated using the Swedish Population Registry, with an update in December 2012. The study was approved by the Ethics Committee of Uppsala University. Informed consent was obtained in accordance with the Declaration of Helsinki.
Definitions
Criteria established by the European LeukemiaNet group were used for definitions of cytogenetic and molecular responses and the progression to AP or BP.18 Sokal, Hasford/Euro, and EUTOS scores were calculated by the data managers at RCC, using published formulas and the reported values for age (Sokal, Euro), spleen size (Sokal, Euro, EUTOS), platelet count (Sokal, Euro), and percentage blood blast cells (Sokal, Euro), eosinophils (Euro), and basophils (Euro, EUTOS).5-7
Statistical methods
Differences in characteristics were tested using either the χ2-test for categorical data or the Mann-Whitney U test for continuous data. In the tables summarizing patient characteristics, missing data are treated as a valid category. Otherwise, in the “Results” section, proportions are based on evaluable cases only. Estimates of overall survival (OS) were calculated, using the Kaplan-Meier method.19 Relative survival (RS) was calculated as the ratio of the observed survival in the study population to the expected survival of the general population.20 The expected mortality rates were assessed from sex-, age-, and period-specific life tables for Sweden. Differences in OS were tested using the log-rank test, and differences in RS were tested using a Poisson regression model.21 All statistical analyses were performed with the statistical software packages Stata and R.22,23
Results
Incidence
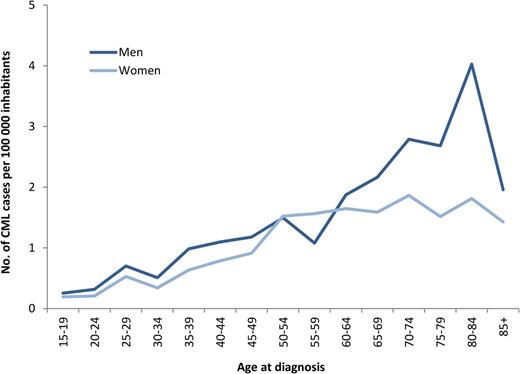
During 2002-2010, a total of 779 newly diagnosed patients with CML (425 men and 354 women), median age 60 years, were reported to the registry (Table 1). This corresponds to an annual incidence of 0.9 per 100 000 inhabitants with a male-to-female ratio of 1.2:1. As shown in Figure 1, the incidence of CML is increasing with age and shows a male preponderance, mainly in the group of patients older than 60 years. There was no difference in incidence between the 6 health care regions (data not shown).
Characteristics of 779 patients diagnosed with CML in Sweden in 2002-2010
| . | Men, n = 425 . | Women, n = 354 . | P* . | Patients living in university hospital catchment areas, n = 285 . | Patients living in other areas, n = 494 . | P* . | Total, N = 779 . |
|---|---|---|---|---|---|---|---|
| Sex, no. (%) | |||||||
| Men | 157 (55.1) | 268 (54.3) | 425 (54.6) | ||||
| Women | 128 (44.9) | 226 (45.7) | .080 | 354 (45.4) | |||
| Age at diagnosis | |||||||
| Median (IQR) | 60 (44-71) | 59 (49-71) | .567 | 60 (46-71) | 59 (46-71) | .902 | 60 (46-71) |
| Age at diagnosis, no. (%) | |||||||
| <50 | 140 (32.9) | 96 (27.1) | 91 (31.9) | 145 (29.4) | 236 (30.3) | ||
| 50-59 | 70 (16.5) | 83 (23.4) | 50 (17.5) | 103 (20.9) | 153 (19.6) | ||
| 60-69 | 90 (21.2) | 74 (20.9) | 60 (21.1) | 104 (21.1) | 164 (21.1) | ||
| 70-79 | 75 (17.6) | 56 (15.8) | 52 (18.2) | 79 (16.0) | 131 (16.8) | ||
| 80+ | 70 (11.8) | 45 (12.7) | .113 | 32 (11.2) | 63 (12.8) | .682 | 95 (12.2) |
| Basis of diagnosis, no. (%) | |||||||
| Karyotyping | 109 (25.6) | 118 (33.3) | 94 (33.0) | 133 (26.9) | 227 (29.1) | ||
| Karyotyping + FISH | 56 (13.2) | 39 (11.0) | 41 (14.4) | 54 (10.9) | 95 (12.2) | ||
| Karyotyping + RT-PCR | 68 (16.0) | 76 (21.5) | 43 (15.1) | 101 (20.4) | 144 (18.5) | ||
| Karyotyping + FISH + RT-PCR | 93 (21.9) | 65 (18.4) | 55 (19.3) | 103 (20.9) | 158 (20.3) | ||
| FISH | 28 (6.6) | 24 (6.8) | 20 (7.0) | 32 (6.5) | 52 (6.7) | ||
| FISH + RT-PCR | 19 (4.5) | 7 (2.0) | 10 (3.5) | 16 (3.2) | 26 (3.3) | ||
| RT-PCR | 35 (8.2) | 18 (5.1) | 15 (5.3) | 38 (7.7) | 53 (6.8) | ||
| Missing | 17 (4.0) | 7 (2.0) | .012 | 7 (2.5) | 17 (3.4) | .230 | 24 (3.1) |
| WHO performance status, no. (%) | |||||||
| WHO 0 | 261 (61.4) | 185 (52.3) | 168 (58.9) | 278 (56.3) | 446 (57.3) | ||
| WHO 1 | 115 (27.1) | 111 (31.4) | 79 (27.7) | 147 (29.8) | 226 (29.0) | ||
| WHO 2-4 | 36 (8.5) | 43 (12.1) | 29 (10.2) | 50 (10.1) | 79 (10.1) | ||
| Missing | 13 (3.1) | 15 (4.2) | .061 | 9 (3.2) | 19 (3.8) | .868 | 28 (3.6) |
| Phase at diagnosis, no. (%) | |||||||
| CP | 386 (90.8) | 331 (93.5) | 264 (92.6) | 453 (91.7) | 717 (92.0) | ||
| AP | 21 (4.9) | 12 (3.4) | 12 (4.2) | 21 (4.3) | 33 (4.2) | ||
| Blast crisis | 12 (2.8) | 7 (2.0) | 7 (2.5) | 12 (2.4) | 19 (2.4) | ||
| Missing | 6 (1.4) | 4 (1.1) | .600 | 2 (0.7) | 8 (1.6) | .801 | 10 (1.3) |
| Spleen size (cm) | |||||||
| Median (IQR) | 0 (0-6) | 0 (0-5) | .170 | 0 (0.6) | 0 (0-6) | .201 | 0 (0-6) |
| Missing, no. (%) | 18 (4.2) | 10 (2.8) | 5 (1.8) | 23 (4.7) | 28 (3.6) | ||
| Hemoglobin (g/L) | |||||||
| Median (IQR) | 114 (101-130) | 112 (96-125) | .016 | 112.5 (100-128) | 114 (99-128) | .992 | 113 (99-128) |
| Missing, no. (%) | 0 (0.0) | 4 (1.1) | 1 (0.4) | 3 (0.6) | 4 (0.5) | ||
| Platelet count (109/L) | |||||||
| Median (IQR) | 391 (236-592) | 512 (342-833) | <.001 | 439.5 (284-693) | 431 (265-715) | .778 | 433 (274-704) |
| Missing, no. (%) | 0 (0.0) | 3 (0.8) | 1 (0.4) | 2 (0.4) | 3 (0.4) | ||
| White blood cell count (109/L) | |||||||
| Median (IQR) | 119 (52.3-110.2) | 106 (44.9-206) | .298 | 103.5 (49-194) | 118 (52-212.5) | .203 | 112 (50-209) |
| Missing, no. (%) | 1 (0.2) | 3 | 1 (0.4) | 3 (0.6) | 4 (0.5) | ||
| Percentage blast cells in blood | |||||||
| Median (IQR) | 1 (0-3.5) | 1 (0-3.4) | .682 | 1 (0-3) | 1 (0-3.5) | .228 | 1 (0-3.4) |
| Missing, no. (%) | 6 (1.4) | 5 (1.4) | 2 (0.7) | 9 (1.8) | 11 (1.4) | ||
| Percentage eosinophils in blood | |||||||
| Median (IQR) | 2 (1-4) | 2 (1-4) | .706 | 2.5 (1-4.5) | 2 (1-4) | .090 | 2 (1-4) |
| Missing, no. (%) | 15 (3.5) | 9 (2.5) | 11 (3.9) | 13 (2.6) | 24 (3.1) | ||
| Percentage basophils in blood | |||||||
| Median (IQR) | 3.5 (1.9-6.9) | 4 (2-7) | .088 | 3.9 (2-7) | 3.7 (2-6.9) | .905 | 3.8 (2-6.9) |
| Missing, no. (%) | 15 (3.5) | 8 (2.3) | 10 (3.5) | 13 (2.6) | 23 (3.0) | ||
| Percentage blast cells in bone marrow | |||||||
| Median (IQR) | 2 (1-4) | 2 (1-4) | .300 | 2 (1-4) | 2 (1-4) | .619 | 2 (1-4) |
| Missing, no. (%) | 47 (11.1) | 40 (11.3) | 30 (10.5) | 57 (11.5) | 87 (11.2) |
| . | Men, n = 425 . | Women, n = 354 . | P* . | Patients living in university hospital catchment areas, n = 285 . | Patients living in other areas, n = 494 . | P* . | Total, N = 779 . |
|---|---|---|---|---|---|---|---|
| Sex, no. (%) | |||||||
| Men | 157 (55.1) | 268 (54.3) | 425 (54.6) | ||||
| Women | 128 (44.9) | 226 (45.7) | .080 | 354 (45.4) | |||
| Age at diagnosis | |||||||
| Median (IQR) | 60 (44-71) | 59 (49-71) | .567 | 60 (46-71) | 59 (46-71) | .902 | 60 (46-71) |
| Age at diagnosis, no. (%) | |||||||
| <50 | 140 (32.9) | 96 (27.1) | 91 (31.9) | 145 (29.4) | 236 (30.3) | ||
| 50-59 | 70 (16.5) | 83 (23.4) | 50 (17.5) | 103 (20.9) | 153 (19.6) | ||
| 60-69 | 90 (21.2) | 74 (20.9) | 60 (21.1) | 104 (21.1) | 164 (21.1) | ||
| 70-79 | 75 (17.6) | 56 (15.8) | 52 (18.2) | 79 (16.0) | 131 (16.8) | ||
| 80+ | 70 (11.8) | 45 (12.7) | .113 | 32 (11.2) | 63 (12.8) | .682 | 95 (12.2) |
| Basis of diagnosis, no. (%) | |||||||
| Karyotyping | 109 (25.6) | 118 (33.3) | 94 (33.0) | 133 (26.9) | 227 (29.1) | ||
| Karyotyping + FISH | 56 (13.2) | 39 (11.0) | 41 (14.4) | 54 (10.9) | 95 (12.2) | ||
| Karyotyping + RT-PCR | 68 (16.0) | 76 (21.5) | 43 (15.1) | 101 (20.4) | 144 (18.5) | ||
| Karyotyping + FISH + RT-PCR | 93 (21.9) | 65 (18.4) | 55 (19.3) | 103 (20.9) | 158 (20.3) | ||
| FISH | 28 (6.6) | 24 (6.8) | 20 (7.0) | 32 (6.5) | 52 (6.7) | ||
| FISH + RT-PCR | 19 (4.5) | 7 (2.0) | 10 (3.5) | 16 (3.2) | 26 (3.3) | ||
| RT-PCR | 35 (8.2) | 18 (5.1) | 15 (5.3) | 38 (7.7) | 53 (6.8) | ||
| Missing | 17 (4.0) | 7 (2.0) | .012 | 7 (2.5) | 17 (3.4) | .230 | 24 (3.1) |
| WHO performance status, no. (%) | |||||||
| WHO 0 | 261 (61.4) | 185 (52.3) | 168 (58.9) | 278 (56.3) | 446 (57.3) | ||
| WHO 1 | 115 (27.1) | 111 (31.4) | 79 (27.7) | 147 (29.8) | 226 (29.0) | ||
| WHO 2-4 | 36 (8.5) | 43 (12.1) | 29 (10.2) | 50 (10.1) | 79 (10.1) | ||
| Missing | 13 (3.1) | 15 (4.2) | .061 | 9 (3.2) | 19 (3.8) | .868 | 28 (3.6) |
| Phase at diagnosis, no. (%) | |||||||
| CP | 386 (90.8) | 331 (93.5) | 264 (92.6) | 453 (91.7) | 717 (92.0) | ||
| AP | 21 (4.9) | 12 (3.4) | 12 (4.2) | 21 (4.3) | 33 (4.2) | ||
| Blast crisis | 12 (2.8) | 7 (2.0) | 7 (2.5) | 12 (2.4) | 19 (2.4) | ||
| Missing | 6 (1.4) | 4 (1.1) | .600 | 2 (0.7) | 8 (1.6) | .801 | 10 (1.3) |
| Spleen size (cm) | |||||||
| Median (IQR) | 0 (0-6) | 0 (0-5) | .170 | 0 (0.6) | 0 (0-6) | .201 | 0 (0-6) |
| Missing, no. (%) | 18 (4.2) | 10 (2.8) | 5 (1.8) | 23 (4.7) | 28 (3.6) | ||
| Hemoglobin (g/L) | |||||||
| Median (IQR) | 114 (101-130) | 112 (96-125) | .016 | 112.5 (100-128) | 114 (99-128) | .992 | 113 (99-128) |
| Missing, no. (%) | 0 (0.0) | 4 (1.1) | 1 (0.4) | 3 (0.6) | 4 (0.5) | ||
| Platelet count (109/L) | |||||||
| Median (IQR) | 391 (236-592) | 512 (342-833) | <.001 | 439.5 (284-693) | 431 (265-715) | .778 | 433 (274-704) |
| Missing, no. (%) | 0 (0.0) | 3 (0.8) | 1 (0.4) | 2 (0.4) | 3 (0.4) | ||
| White blood cell count (109/L) | |||||||
| Median (IQR) | 119 (52.3-110.2) | 106 (44.9-206) | .298 | 103.5 (49-194) | 118 (52-212.5) | .203 | 112 (50-209) |
| Missing, no. (%) | 1 (0.2) | 3 | 1 (0.4) | 3 (0.6) | 4 (0.5) | ||
| Percentage blast cells in blood | |||||||
| Median (IQR) | 1 (0-3.5) | 1 (0-3.4) | .682 | 1 (0-3) | 1 (0-3.5) | .228 | 1 (0-3.4) |
| Missing, no. (%) | 6 (1.4) | 5 (1.4) | 2 (0.7) | 9 (1.8) | 11 (1.4) | ||
| Percentage eosinophils in blood | |||||||
| Median (IQR) | 2 (1-4) | 2 (1-4) | .706 | 2.5 (1-4.5) | 2 (1-4) | .090 | 2 (1-4) |
| Missing, no. (%) | 15 (3.5) | 9 (2.5) | 11 (3.9) | 13 (2.6) | 24 (3.1) | ||
| Percentage basophils in blood | |||||||
| Median (IQR) | 3.5 (1.9-6.9) | 4 (2-7) | .088 | 3.9 (2-7) | 3.7 (2-6.9) | .905 | 3.8 (2-6.9) |
| Missing, no. (%) | 15 (3.5) | 8 (2.3) | 10 (3.5) | 13 (2.6) | 23 (3.0) | ||
| Percentage blast cells in bone marrow | |||||||
| Median (IQR) | 2 (1-4) | 2 (1-4) | .300 | 2 (1-4) | 2 (1-4) | .619 | 2 (1-4) |
| Missing, no. (%) | 47 (11.1) | 40 (11.3) | 30 (10.5) | 57 (11.5) | 87 (11.2) |
IQR, interquartile range; WHO, World Health Organization.
P value for the null hypothesis of no difference between the sexes or the patients’ area of residence.
Age- and sex-specific incidence of CML per 100 000 inhabitants, year 2002-2010.
Age- and sex-specific incidence of CML per 100 000 inhabitants, year 2002-2010.
Patient characteristics at diagnosis
The diagnosis of CML was confirmed by karyotyping, FISH, and/or RT-PCR in all but 24 cases. The latter were mainly elderly patients (median age, 82 years). Details regarding blood counts and spleen size are shown in Table 1. A large majority, 717 patients (93%), were diagnosed as being in CP; 33 (4%) patients were in AP, and 19 (2%) patients were in BP, with median ages of 60, 54, and 60 years, respectively. As shown in Table 2, the proportion of CP patients scored as “high-risk group” was 32%, 17%, and 14%, according to the Sokal, Euro, and EUTOS scores, respectively.
Prognostic scores of patients with confirmed CML in CP at diagnosis
| . | Men, n = 373 . | Women, n = 326 . | P* . | Patients living in university hospital catchment areas, n = 257 . | Patients living in other areas, n = 442 . | P* . | Total, N = 699 . |
|---|---|---|---|---|---|---|---|
| Sokal score, no. (%) | |||||||
| Low risk | 94 (25.2) | 62 (19.0) | 64 (24.9) | 92 (20.8) | 156 (22.3) | ||
| Intermediate risk | 164 (44.0) | 136 (41.7) | 112 (43.6) | 188 (42.5) | 300 (42.9) | ||
| High risk | 104 (27.9) | 116 (35.6) | 78 (30.4) | 142 (32.1) | 220 (31.5) | ||
| Missing | 11 (2.9) | 12 (3.7) | .080 | 3 (1.2) | 20 (4.5) | .072 | 23 (3.3) |
| Hasford score, no. (%) | |||||||
| Low risk | 127 (34.0) | 85 (26.1) | 84 (32.7) | 128 (29.0) | 212 (30.3) | ||
| Intermediate risk | 172 (46.1) | 162 (49.7) | 120 (46.7) | 214 (48.4) | 334 (47.8) | ||
| High risk | 56 (15.0) | 63 (19.3) | 44 (17.1) | 75 (17.0) | 119 (17.0) | ||
| Missing | 18 (4.8) | 16 (4.9) | .111 | 9 (3.5) | 25 (5.7) | .494 | 34 (4.9) |
| EUTOS score, no. (%) | |||||||
| Low risk | 298 (79.9) | 271 (83.1) | 210 (81.7) | 359 (81.2) | 569 (81.4) | ||
| High risk | 57 (15.3) | 41 (12.6) | 38 (14.8) | 60 (13.6) | 98 (14.0) | ||
| Missing | 18 (4.8) | 14 (4.3) | .538 | 9 (3.5) | 23 (5.2) | .548 | 32 (4.6) |
| . | Men, n = 373 . | Women, n = 326 . | P* . | Patients living in university hospital catchment areas, n = 257 . | Patients living in other areas, n = 442 . | P* . | Total, N = 699 . |
|---|---|---|---|---|---|---|---|
| Sokal score, no. (%) | |||||||
| Low risk | 94 (25.2) | 62 (19.0) | 64 (24.9) | 92 (20.8) | 156 (22.3) | ||
| Intermediate risk | 164 (44.0) | 136 (41.7) | 112 (43.6) | 188 (42.5) | 300 (42.9) | ||
| High risk | 104 (27.9) | 116 (35.6) | 78 (30.4) | 142 (32.1) | 220 (31.5) | ||
| Missing | 11 (2.9) | 12 (3.7) | .080 | 3 (1.2) | 20 (4.5) | .072 | 23 (3.3) |
| Hasford score, no. (%) | |||||||
| Low risk | 127 (34.0) | 85 (26.1) | 84 (32.7) | 128 (29.0) | 212 (30.3) | ||
| Intermediate risk | 172 (46.1) | 162 (49.7) | 120 (46.7) | 214 (48.4) | 334 (47.8) | ||
| High risk | 56 (15.0) | 63 (19.3) | 44 (17.1) | 75 (17.0) | 119 (17.0) | ||
| Missing | 18 (4.8) | 16 (4.9) | .111 | 9 (3.5) | 25 (5.7) | .494 | 34 (4.9) |
| EUTOS score, no. (%) | |||||||
| Low risk | 298 (79.9) | 271 (83.1) | 210 (81.7) | 359 (81.2) | 569 (81.4) | ||
| High risk | 57 (15.3) | 41 (12.6) | 38 (14.8) | 60 (13.6) | 98 (14.0) | ||
| Missing | 18 (4.8) | 14 (4.3) | .538 | 9 (3.5) | 23 (5.2) | .548 | 32 (4.6) |
P value for the null hypothesis of no difference between the sexes or the patients’ area of residence.
First-line treatment, time trends
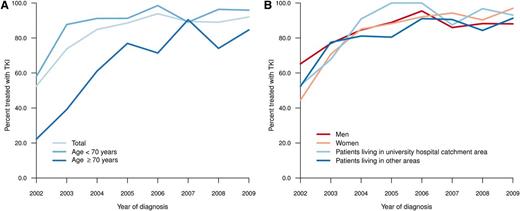
Only 18% of all patients were enrolled in clinical trials of first-line treatment (2002-2004, 7%; 2005-2007, 23%; 2008-2010, 22%). A small and decreasing proportion of elderly patients received first-line treatment with the intention of palliation (ie, not including TKIs or up-front HSCT), as opposed to the intention to achieve CCgR or MMR (data not shown). During the first years of the study, only a minority (20%-40%) of patients aged 70 years or older received a TKI as their main first-line treatment (Figure 2A). However, in 2007-2009, 94% (<70 years), 85% (70-79 years), and 79% (80+ years) of patients were treated with TKI within 12 months from diagnosis. Follow-up data at 12 months after diagnosis were available from 589 patients. Only 29 (5%) of these patients, of whom 19 were in CP at diagnosis, underwent HSCT as part of first-line treatment in this time.
Time trends in percentage of patients treated with TKI by age (A) and by residence (university hospital catchment area vs other area) and sex (B).
Time trends in percentage of patients treated with TKI by age (A) and by residence (university hospital catchment area vs other area) and sex (B).
Response evaluation at 12 months after diagnosis
At the 12-month follow-up, only 16 (3%) of 531 evaluable patients had transformed from CP to either AP (n = 6) or BP (n = 10). Evaluation regarding adherence to the Swedish national CML management guidelines24 showed that 463 (91%) of 511 patients treated with nonpalliative intention (TKI or HSCT) were subjected to recommended karyotyping and/or quantitative PCR at the 1-year timeline. A major cytogenetic response (MCgR) was obtained in 86%, and a CCgR in 66%, of these patients. During the period between 2007 and 2009, BCR-ABL1 transcript levels were also reported at the 1-year time point, showing a MMR in 36% (62/172) of the patients.
Survival by age, sex, and prognostic subgroups
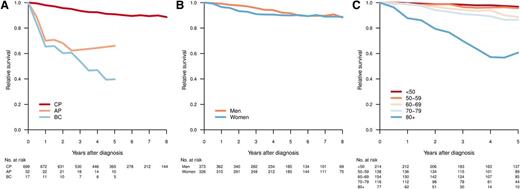
With a median follow-up of 61 months, 5-year OS was 81% (95% confidence interval [CI], 78%-84%) for all registered CML patients and 83% (95% CI, 80%-86%) for those diagnosed as being in CP. Figure 3 shows the RS of patients with CML diagnosed in CP, AP, and BP, respectively (median follow-up, 61 months). As expected, those in AP or BP had an inferior outcome (Figure 3A). Among CP patients, men had a trend toward better RS, but this did not reach statistical significance (Figure 3B). Notably, for CP patients younger than 60 years, the RS at 5 years was close to 1 (0.97 [95% CI, 0.93-0.99] for age <50 years and 0.96 [95% CI, 0.89-0.99] for age 50-59 years; Figure 3C). In contrast, there was an excess mortality in elderly CML patients, and in particular in those aged 80 years or older at diagnosis (5-year RS, 0.61; 95% CI, 0.39-0.82).
Relative survival by disease phase at diagnosis (A), sex (CP) (B), and age group (CP) (C). Relative survival was calculated as the ratio of the observed survival in the study population to the expected survival of the general population.
Relative survival by disease phase at diagnosis (A), sex (CP) (B), and age group (CP) (C). Relative survival was calculated as the ratio of the observed survival in the study population to the expected survival of the general population.
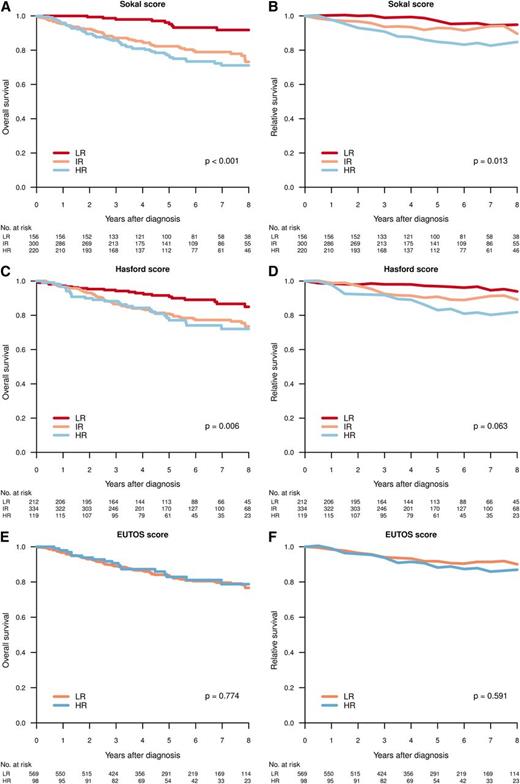
Figure 4 shows the OS and RS by Sokal, Euro, and EUTOS scores in patients diagnosed in CP. A difference in survival (HR vs IR/LR) was predicted by Sokal and Euro/Hasford score, but not by EUTOS score.
Overall (A,C,E) and relative (B,D,F) survival for patients in CP at diagnosis, per Sokal (A-B), Hasford (C-D), and EUTOS (E-F) score. Differences in survival tested by log-rank test (OS) and Poisson regression model (RS).
Overall (A,C,E) and relative (B,D,F) survival for patients in CP at diagnosis, per Sokal (A-B), Hasford (C-D), and EUTOS (E-F) score. Differences in survival tested by log-rank test (OS) and Poisson regression model (RS).
Decentralized clinical management of CML
A total of 62 different hospitals reported at least a single newly diagnosed CML patient during the entire 9-year period under study. Forty-nine hospitals reported on average only 2 or fewer new CML cases each year. A total of 51% of all patients reported during the period were diagnosed at any of the 7 university hospital hematology units, whereas 5% were referred to such a unit from a smaller hospital after diagnosis for further treatment and follow-up. Patients diagnosed at university hospitals were more often included in clinical trials up front than those diagnosed at county hospitals (24% vs 11%; P < .001).
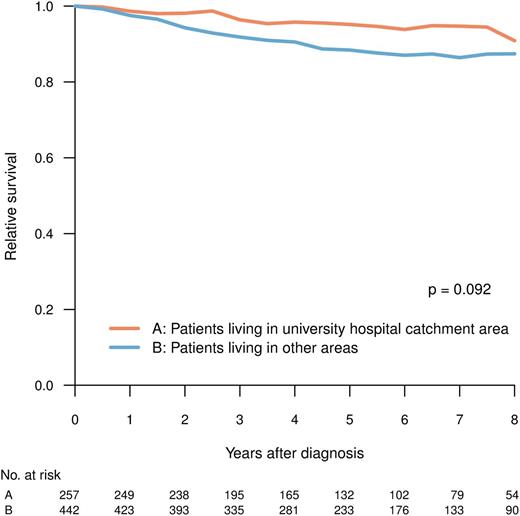
Patients living in university and nonuniversity hospital catchment areas, respectively, were comparable with respect to age, clinical characteristics, and prognostic scores (Tables 1 and 2), but they differed in the proportion of patients who, during the period between 2004 and 2009, received TKI as first-line treatment (Figure 2B; university vs nonuniversity hospital catchment area, 92% vs 86%; P = .048, χ2-test). The latter difference was mainly caused by the slower implementation of imatinib in the elderly population in nonuniversity hospital areas during the study (data not shown). However, there was no statistically significant difference in RS at 5 years (median follow-up, 61 months) between patients living and not living in university hospital catchment areas (P = .092) (Figure 5).
RS for patients in CP at diagnosis, by whether or not the patient resided in the catchment area of any of the Swedish university hospitals.
RS for patients in CP at diagnosis, by whether or not the patient resided in the catchment area of any of the Swedish university hospitals.
Discussion
The incidence of CML, as shown by our study, is lower than that suggested in a recent publication from the Surveillance Epidemiology and End Results Program, which reported an annual incidence of 1.6 per 100 000 based on cases diagnosed in 2006-2010 from 18 program geographic areas (http://seer.cancer.gov/statfacts/html/cmyl.html). However, several epidemiological studies from the United Kingdom, Germany, and the United States have shown an annual incidence in the range of 0.7 to 1.3 per 100 000.11,25-28 Previous reports from the Swedish Cancer Registry have shown an incidence close to 1.0 per 100 000.1,29 Possible reasons for these “discrepancies” include a true difference resulting from different ethnic populations, underreporting or, indeed, overreporting resulting from the inclusion of patients with Philadelphia chromosome-negative myeloproliferative disorders. Notably, in the Swedish CML registry material, 97% of all patients have a cytogenetically confirmed diagnosis. Underreporting to our registry is unlikely, as in Sweden, all CML patients are treated within the public health care system, in which clinicians are strongly encouraged to report all cases of newly diagnosed cases to the CML registry. Moreover, pathologists and clinicians are obliged by law to report all new cases of cancer (eg, CML) to the Swedish Cancer Registry, which makes it possible for the data managers at the RCCs to identify and request reports from cases missing in the CML registry. The completeness of the Swedish Cancer Registry has been shown by previous publications.30
After the introduction of imatinib and, more recently, second-generation TKIs, the prognosis of CML has improved dramatically. According to results from clinical trials, as well as large, single-institution studies, patients diagnosed in CP and treated with imatinib have a long-term (5 years or more) OS of 83% to 88%.3,31 It has been argued that patients treated outside clinical trials may have an inferior outcome.12 However, using data from the population-based Swedish CML registry, including 97% of all CML patients diagnosed at more than 60 different hospitals during 2002-2010, we here show a 5-year OS in CP of 83% (95% CI, 0.80-0.86). This compares favorably with that observed in the above-cited study populations when taking into account that there was no upper age limit in our cohort and that the median age was 60 years (ie, 12-14 years higher than in the other studies mentioned). Moreover, the rate of cytogenetic response after 12 months of therapy found in our population-based cohort (MCgR, 86%; CCgR, 66%) was at least as good as that observed in a large, single-institution study of imatinib-treated patients (MCgR, 71%; CCgR, 57%).2 Notably, the vast majority of our patients received imatinib as primary treatment, whereas only 5% were transplanted within the first year after diagnosis. As expected, patients with AP or BP had an inferior outcome.
Corroborating the findings by Björkholm et al, who used population-based data from the Swedish Cancer Registry,1 the RS in patients younger than 60 years was shown to be almost identical to that of a normal age-matched population. Compared with the Swedish Cancer Registry, our CML registry contains considerably more detailed data, allowing survival analyses of subgroups of patients defined by cytogenetic or molecular diagnostics, as well as analyzing outcomes by disease stage and prognostic subgroups (see following). Considering that median follow-up was 61 months, it remains to be shown whether the excellent prognosis in younger CML patients holds true even with a more extended follow-up time (>10 years).
However, in elderly CML patients, the RS is still significantly decreased. This is particularly evident for those older than 80 years who had a 5-year RS of only 0.61 (95% CI, 0.39-0.82). Although the Swedish CML registry lacks detailed information on treatments received by individual patients, the physicians reported first-line therapy given during the first year after diagnosis. Notably, our data show that during 2002-2004, despite recommendations in our CML guidelines,24 the majority of elderly patients still received non-imatinib-containing regimens as first-line treatment. A continuous change was seen during the study period, and from 2006, a clear majority of patients older than 70 years did receive TKIs up front, although this patient cohort was still less likely to be treated with TKIs than younger patients. Thus, the inferior survival results in the elderly CML patients observed in our study may indeed be a result of an underimplementation of TKI therapy during the early period of this study, although other explanations such as poor compliance or tolerance to TKIs cannot be excluded.32,33 Future analyses will show whether the increasing use of imatinib, or the introduction of second-generation TKIs (eg, nilotinib and dasatinib), will further reduce the excess CML-related mortality seen in the elderly population.
Interestingly, in our unselected, population-based registry cohort, we found that the Sokal score, but not the recently launched EUTOS score, predicted OS and RS in patients diagnosed in CP. Sokal and Euro scores were originally developed for baseline stratification of CML patients treated with conventional chemotherapy and α-interferon, respectively.5-7 In the modern TKI era, response-related, rather than baseline prognostic, factors seem to be more important, particularly for those receiving treatment with potent second-generation TKIs.34,35 Nevertheless, several studies including patients receiving first-line treatment with imatinib have shown that the classical baseline scores predict CCgR (Sokal, Euro), and even OS (Sokal).31,36 Moreover, in a recent, reports from 5 different national trial groups, including 2060 patients treated with imatinib within clinical trials, the EUTOS score was found to be superior to Sokal and Euro score in predicting CCgR within 18 months and was also found to predict 5-year progression-free survival.7 However, comparing the results of our study and those of the European LeukemiaNet group, one should note that our primary outcome measure was RS, rather than CCgR or progression-free survival. Furthermore, our analysis also included a small minority of patients receiving non-imatinib-based primary treatments. However, even when we restricted our analysis to only the imatinib-treated patients, there was still no significant difference in survival between the high-risk and low-risk EUTOS groups (data not shown). Interestingly, 2 relatively large, single-institution studies were recently unable to confirm the prognostic value of the EUTOS score.8,9 Clearly, further and more detailed analyses of the validity and clinical relevance of this and other published baseline prognostic scores are needed.
In Sweden, CML treatment is decentralized, as shown by the fact that the diagnosis of CML was registered by 62 different hospitals. Notably, half of the patients were initially diagnosed and reported from nonuniversity units, and 18% of all registered patients were included in clinical trials up front. Although geographical considerations and the relative simplicity of guideline-based treatment with TKIs make treatment at the local hospital a reasonable option, there may still be an argument for centralizing the care of CML patients to larger, more specialized units. Thus, CML implies long-term treatment with expensive drugs, ideally within the framework of clinical trials and good facilities for cytogenetic and molecular monitoring, as well as continuously updated clinical expertise to make correct decisions regarding response assessment and switching to second-line therapy. Indeed, previous reports from the Swedish CML registry indicated suboptimal adherence to established guidelines with respect to cytogenetic evaluation at 12 months postdiagnosis, as well as a clear underuse of imatinib in the elderly population.37
Furthermore, recent publications from the Slovakian CML registry (Chronic MyEloid LeukaemIA; CAMELIA) and from the US population-based database (the Surveillance Epidemiology and End Results Program) both concluded that centralized care is important for achieving results comparable with those of clinical trials.10,12 However, comparing patients living in university hospital catchment areas with those living outside, we found only a trend (not significant) indicating better RS for those in the first category. This trend may reflect that implementation of TKIs in the elderly patient population during the earliest part of study period was slower in nonuniversity hospitals, although we cannot exclude other explanations such as differences in the socioeconomic composition of the cohorts treated at different hospital categories.38 Thus, decentralized care for CML patients by dedicated clinicians at local hospitals, having the option to refer to or consult CML specialists in larger units, may well reach an acceptable level of clinical CML management. Nevertheless, we believe that the continuous evaluation of adherence to CML guidelines and treatment results by a well-established quality-of-care registry is of key importance in a decentralized health care system of this type.
In summary, patients up to ages 60-70 years with CML diagnosed in CP, and treated within a decentralized health care setting, had a 5-year survival that was close to that of the age-matched general population, whereas elderly patients still had an inferior outcome. Treatment results, including OS, were on par with those obtained in recently published clinical trials and at large institutions. Sokal, but not EUTOS, score predicted relative survival. Future studies from the Swedish CML registry will focus on the use of second-generation TKIs in different age groups, outcome in terms of molecular response, more detailed validation of prognostic scores, and continuous evaluation of adherence to established guidelines on the management of patients with CML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all Swedish hematologists who have carefully reported CML patients to the CML Registry. The authors also appreciate the work of data managers at the respective RCCs.
Authorship
Contribution: M.H., L.S., and J.R. provided conception and design, patient material, data collection, data analysis, data interpretation, and manuscript writing; F.S. provided data analysis and interpretation, statistical methodology, and manuscript writing; K.H. provided data collection, data analysis, data interpretation, and manuscript writing; B.S. provided conception and design and manuscript writing; M. Björeman, M. Björkholm, M. Brune, A.D., M.E., S.L., P.L., C.M., B.M., K.M.-E., L.O., U.O.-S., A.S., and H.W. provided patient material, data collection, and manuscript writing; and all authors contributed with critical revision of the manuscript.
Conflict-of-interest disclosure: J.R. has received honoraria from Bristol-Myers Squibb and honoraria and research support from Novartis. M. Björkholm has received research support from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Martin Höglund, Division of Hematology (50C), Uppsala University Hospital, SE-751 85 Uppsala, Sweden; e-mail: martin.hoglund@medsci.uu.se.
References
Author notes
L.S. and J.R. contributed equally.