Key Points
Patients with subsegmental PE appear to mimic those with more proximally located PE regarding their VTE risk profile and clinical outcome.
Patients with subsegmental PE significantly differ from patients without PE in terms of both VTE risk profile and clinical outcome.
Abstract
The clinical significance of subsegmental pulmonary embolism (SSPE) remains to be determined. This study aimed to investigate whether SSPE forms a distinct subset of thromboembolic disease compared with more proximally located pulmonary embolism (PE). We analyzed 3728 consecutive patients with clinically suspected PE. SSPE patients were contrasted to patients with more proximal PE and to patients in whom suspected PE was ruled out, in regards of the prevalence of thromboembolic risk factors and the 3-month risks of recurrent venous thromboembolism (VTE) and mortality. PE was confirmed in 748 patients, of whom 116 (16%) had SSPE; PE was ruled out in 2980 patients. No differences were seen in the prevalence of VTE risk factors, the 3-month risk of recurrent VTE (3.6% vs 2.5%; P = .42), and mortality (10.7% vs 6.5%; P = .17) between patients with SSPE and those with more proximal PE. When compared with patients without PE, aged >60 years, recent surgery, estrogen use, and male gender were found to be independent predictors for SSPE, and patients with SSPE were at an increased risk of VTE during follow-up (hazard ratio: 3.8; 95% CI: 1.3-11.1). This study indicates that patients with SSPE mimic those with more proximally located PE in regards to their risk profile and clinical outcome.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1329.
Disclosures
The authors, Associate Editor David Lillicrap, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Compare the risk profile of patients with subsegmental pulmonary embolism (SSPE) with that of patients with segmental or more proximal pulmonary embolism (PE), based on a clinical study.
Compare the risk profile of patients with SSPE with that of patients without PE.
Describe the clinical outcomes of patients with SSPE.
Release date: August 15, 2013; Expiration date: August 15, 2014
Introduction
The introduction of multi-detector computed tomographic pulmonary angiography (CTPA) has considerably advanced the radiological visualization of pulmonary embolism (PE) and its diagnostic accuracy has been demonstrated to be robust enough to serve as the single imaging test in the diagnostic work-up of patients with suspected PE.1 Compared with previously used imaging techniques to detect PE, multi-detector CTPA allows better visualization of peripheral pulmonary arteries.2 As a consequence of the widespread use of these scanners as first-line imaging tools to establish or rule out acute PE, small peripheral emboli limited to the subsegmental pulmonary arteries are increasingly being detected. The proportion of this so-called isolated subsegmental PE (SSPE) detected on CTPA varies between 4% and 27%.3-5
With this increasing incidence of SSPE diagnoses that would probably have gone undetected and thus left untreated with former imaging techniques, physicians started to question the clinical relevance of these findings.6 The prognostic implications of SSPE are, however, uncertain, because the clinical outcome of these patients has been investigated in few studies. It therefore remains unclear whether a diagnosis of SSPE deserves the same therapeutic approach as PE located in segmental or more proximal pulmonary arteries.7 Recently, some evidence suggested that patients with SSPE may have a favorable clinical outcome, even without prescribing anticoagulant therapy.8,9
To investigate whether SSPE could be considered as a distinctive subset of venous thromboembolic disease or even as a prognostically insignificant finding, we compared patients with SSPE to patients with PE located in more proximally located pulmonary arteries and patients in whom PE was clinically suspected but ruled out regarding their thromboembolic risk factors, clinical signs and symptoms, and short-term clinical outcome in terms of recurrent venous thromboembolism (VTE), bleeding complications, and mortality.
Methods
Study population
We used the combined data of 2 prospective outcome studies in which consecutive patients with clinically suspected PE had been included. The first study was a large, prospective, management study including 3306 consecutive patients10 with an aim to evaluate a diagnostic algorithm consisting of the Wells rule,11 D-dimer testing, and CTPA. The exclusion criteria for this study were: treatment with therapeutic doses of unfractionated or low-molecular–weight heparin for >24 hours; life expectancy <3 months; pregnancy; geographic inaccessibility for follow-up; age <18 years; allergy to IV contrast agents; or hemodynamic instability (defined as a systolic blood pressure <90 mm Hg or clinical signs and symptoms of shock). The institutional review boards of all participating hospitals approved the study protocol, and informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
The decision regarding the presence or absence of PE was made by trained attending radiologists who were blinded to any specific patient clinical information. The pulmonary arteries were evaluated down to and including the subsegmental arteries. Embolus localization was classified as central, segmental, or subsegmental. Isolated SSPE was defined as a PE that occurred in a subsegmental branch but no larger order of vessels.12 The SSPE could involve one or more than one subsegmental artery. All patients with confirmed PE were initially treated with subcutaneous body weight-adjusted therapeutic doses of LMWH for a minimum of 5 days or IV unfractionated heparin aimed at an activated partial thromboplastin time between 1.5 and 2 times the baseline value, followed by vitamin K antagonists aimed at an international normalized ratio of 2.0 to 3.0 for a period of 6 months.
The second cohort included 463 consecutive patients with suspected PE, with an aim to identify predictors for the outcome of patients with PE.13 All patients provided written informed consent. The exclusion criteria for this study were: impossibility for follow-up, age <18 years, pregnancy, allergy to IV contrast agents, or hemodynamic instability at initial presentation. All patients with a likely clinical probability by the Wells rule (>4 points total) and/or an abnormal D-dimer test (>500 ng/mL) underwent multi-detector row CTPA during breath-hold inspiration. The presence of PE was defined as at least one filling defect in the pulmonary artery tree. The method of Qanadli et al14 was used to quantify the degree of pulmonary arterial obstruction, and the largest pulmonary artery involved, ie, central, segmental, or subsegmental, was recorded. Isolated SSPE was defined as PE that occurred in a subsegmental branch but no larger order of vessels. All patients in whom PE was confirmed were treated similarly to patients with PE in the first cohort.
Risk factors
We investigated the influence of the following thromboembolic risk factors that were recorded in both studies at baseline: age; sex; hospitalization status; immobilization for at least 3 days within the past four weeks; paralysis, pareses, or leg plaster within the past month; major surgery within the past month; a history of VTE; estrogen use; heart failure (defined as New York Heart Association functional class II-IV for which specific therapy was administered); chronic obstructive pulmonary disease (COPD); and active malignancy, defined as any malignancy with ongoing treatment or treatment within the past 6 months, or malignancies in palliative stages.
Outcome
For this comparative analysis, the cohort was stratified into 3 groups: 1) patients with isolated SSPE; 2) patients with segmental or more proximal PE; and 3) patients in whom clinically suspected PE was ruled out. These 3 groups were compared for the incidence of symptomatic (recurrent) VTE, incidence of major bleeding complications, and incidence of all-cause mortality during 3 months of follow-up. VTE during follow-up was defined as an objective diagnosis of recurrent PE or deep vein thrombosis (DVT), or death in which PE could not be ruled out as a contributing cause. The objective criterion for the diagnosis of recurrent PE was a new intraluminal filling defect on CTPA or pulmonary angiography; a new high-probability perfusion defect on ventilation-perfusion scan; a new nondiagnostic lung scan accompanied by documentation of DVT by ultrasonography or venography; or confirmation of a new PE at autopsy. A diagnosis of (recurrent) DVT had to be confirmed by compression ultrasonography or contrast venography.15
Major bleeding was defined as fatal bleeding, symptomatic bleeding in a critical area or organ, clinically overt bleeding causing a fall in hemoglobin level of at least 20 g L−1 (1.24 mmol L−1) or more, or leading to transfusion of 2 or more units of whole blood or red cells.16 An independent adjudication committee reviewed and classified all suspected outcome events. Mortality was classified as caused by PE in case of confirmation at autopsy, in case of an objective test demonstrating PE prior to death, or if PE could not be confidently ruled out as the cause of death.
Statistical analysis
Differences in patient characteristics between strata were tested for statistical significance using the χ-square test for categorical data and the Student t test for continuous variables. P values < .05 were considered statistically significant.
Logistic regression analyses were performed to analyze the association between potential risk factors for VTE and the presence and location of the PE (ie, odds ratios [ORs] were calculated for “no PE” vs SSPE and SSPE vs “more proximally localized PE”). Any variable achieving a P value < .10 was included in a multivariate logistic regression model.
The method of Kaplan and Meier was used to estimate the cumulative probability of recurrent VTE and mortality, and the log-rank test was used to compare the groups for statistical differences. The patients were censored at time of event, time of death, or time of the end of follow-up, whichever came first. A Cox proportional hazard model was used to derive hazard ratios (HRs). HRs for recurrent VTE were adjusted for age, sex, malignant disease, and previous VTE. HRs for mortality were adjusted for age, gender, active malignancy, COPD, and heart failure. SPSS, version 20 (SPSS Inc., Chicago, IL), was used for all analyses.
Results
Patient characteristics
The combined cohort consisted of a total of 3769 patients with suspected PE. A total of 2688 patients underwent CTPA at baseline, based on either a likely clinical decision rule or abnormal D-dimer test. PE was confirmed in 789 of the 3769 patients (21%). Localization of PE was not determined in 41 (5.2%) patients, and those were excluded from further analysis. Of the remaining 748 patients with PE, 116 (15.5%) had a diagnosis of isolated SSPE, leaving 632 patients who had PE localized in a segmental or more proximal pulmonary artery. In 2980 patients, PE was ruled out either at the basis of an unlikely clinical probability and a normal D-dimer test result or on the basis of CTPA.
The mean age of patients with SSPE was 56 years, compared with 57 years for patients with PE localized in segmental or more proximal arteries and 52 years for patients in whom PE was ruled out (Table 1). In these 3 groups, 55%, 49%, and 41% of the patients, respectively, were male. The prevalence of a likely clinical probability (Wells score >411 ) was lower for SSPE patients than for segmental or more proximal PE patients (50% vs 61%; P = .02). On the other hand, when compared with patients without PE, patients with SSPE were more frequently classified as having a likely clinical probability (50% vs 27%; P < .001).
Baseline characteristics
| . | SSPE . | Proximal PE* . | PE ruled out . | P value . | P value . |
|---|---|---|---|---|---|
| (n = 116) . | (n = 632) . | (n = 2980) . | SSPE vs proximal PE . | SSPE vs PE ruled out . | |
| Age, mean ± SD | 56 ± 17 | 57 ± 18 | 52 ± 18 | .46 | .02 |
| Age >60, n (%) | 50 (43.1) | 301 (47.6) | 1004 (33.7) | .37 | .04 |
| Male sex, n (%) | 64 (55.2) | 309 (48.9) | 1212 (40.7) | .23 | .002 |
| Outpatients, n (%) | 87 (75) | 496 (78.5) | 2431 (81.6) | .41 | .07 |
| VTE risk factors | |||||
| Immobilization, n (%) | 20 (17.2) | 108 (17.1) | 273 (9.2) | .97 | .004 |
| Paralysis, pareses, or recent leg plaster, n (%) | 5 (4.3%) | 37 (5.9%) | 63 (2.1) | .51 | .11 |
| Previous VTE, n (%) | 17 (14.7) | 128 (20.3) | 395 (13.3) | .16 | .66 |
| Recent surgery, n (%) | 15 (12.9) | 72 (11.4) | 155 (5.2) | .64 | <.001 |
| Active malignancy, n (%) | 21 (18.1) | 113 (17.9) | 347 (11.6) | .95 | .04 |
| Estrogen use, women, n (%) | 15 (30.0) | 93 (29.2) | 360 (20.7) | .91 | <.001 |
| Clinical signs and symptoms | |||||
| Duration of complaints, d (median, range) | 2 (0-90) | 3 (0-90) | 2 (0-120) | NA | NA |
| Suspected DVT, n (%) | 10 (8.6) | 81 (12.8) | 90 (3.0) | .08 | .002 |
| Hemoptysis, n (%) | 10 (8.6) | 41 (6.5) | 100 (3.4) | .65 | .03 |
| Clinical probability (Wells score11 ) | |||||
| Unlikely, n (%) | 58 (50) | 244 (38.6) | 2179 (73.1) | .02 | <.001 |
| Likely, n (%) | 58 (50) | 388 (61.4) | 800 (26.9) | ||
| Comorbidities | |||||
| COPD, n (%) | 11 (9.5) | 55 (8.7) | 337 (11.3) | .78 | .55 |
| Heart failure, n (%) | 10 (8.6) | 30 (4.7) | 234 (7.9) | .09 | .75 |
| . | SSPE . | Proximal PE* . | PE ruled out . | P value . | P value . |
|---|---|---|---|---|---|
| (n = 116) . | (n = 632) . | (n = 2980) . | SSPE vs proximal PE . | SSPE vs PE ruled out . | |
| Age, mean ± SD | 56 ± 17 | 57 ± 18 | 52 ± 18 | .46 | .02 |
| Age >60, n (%) | 50 (43.1) | 301 (47.6) | 1004 (33.7) | .37 | .04 |
| Male sex, n (%) | 64 (55.2) | 309 (48.9) | 1212 (40.7) | .23 | .002 |
| Outpatients, n (%) | 87 (75) | 496 (78.5) | 2431 (81.6) | .41 | .07 |
| VTE risk factors | |||||
| Immobilization, n (%) | 20 (17.2) | 108 (17.1) | 273 (9.2) | .97 | .004 |
| Paralysis, pareses, or recent leg plaster, n (%) | 5 (4.3%) | 37 (5.9%) | 63 (2.1) | .51 | .11 |
| Previous VTE, n (%) | 17 (14.7) | 128 (20.3) | 395 (13.3) | .16 | .66 |
| Recent surgery, n (%) | 15 (12.9) | 72 (11.4) | 155 (5.2) | .64 | <.001 |
| Active malignancy, n (%) | 21 (18.1) | 113 (17.9) | 347 (11.6) | .95 | .04 |
| Estrogen use, women, n (%) | 15 (30.0) | 93 (29.2) | 360 (20.7) | .91 | <.001 |
| Clinical signs and symptoms | |||||
| Duration of complaints, d (median, range) | 2 (0-90) | 3 (0-90) | 2 (0-120) | NA | NA |
| Suspected DVT, n (%) | 10 (8.6) | 81 (12.8) | 90 (3.0) | .08 | .002 |
| Hemoptysis, n (%) | 10 (8.6) | 41 (6.5) | 100 (3.4) | .65 | .03 |
| Clinical probability (Wells score11 ) | |||||
| Unlikely, n (%) | 58 (50) | 244 (38.6) | 2179 (73.1) | .02 | <.001 |
| Likely, n (%) | 58 (50) | 388 (61.4) | 800 (26.9) | ||
| Comorbidities | |||||
| COPD, n (%) | 11 (9.5) | 55 (8.7) | 337 (11.3) | .78 | .55 |
| Heart failure, n (%) | 10 (8.6) | 30 (4.7) | 234 (7.9) | .09 | .75 |
SD, standard deviation.
Defined as PE localized in a segmental or central pulmonary artery.
Thromboembolic risk factors
No significant differences were found in the prevalence of thromboembolic risk factors between patients with SSPE and patients with segmental or more proximal PE (Tables 1 and 2). When compared with patients without PE, the proportions of patients with malignancy (18% vs 12%), immobility (17% vs 9%), recent surgery 13% vs 5%), and estrogen use (30% vs 20%) were higher among patients with SSPE. On multivariate analysis, age >60 years (OR 1.6; 95% CI: 1.07-2.42), recent surgery (OR 2.3; 1.23-4.20), estrogen use (OR 2.5; 1.34-4.81), and male gender (OR 2.1; 1.38-3.32) remained significantly associated with SSPE (Table 2).
Risk factors for SSPE on multivariate analysis
| . | SSPE vs PE excluded . | SSPE vs proximal PE . |
|---|---|---|
| OR (95% CI) | ||
| Age >60 y | 1.6 (1.1-2.4)* | 0.9 (0.6-1.4) |
| Male sex | 2.1 (1.4-3.2)* | 0.8 (0.5-1.2) |
| Immobilization | 1.6 (0.9-2.7) | 0.9 (0.5-1.6) |
| Previous VTE | 1.4 (0.8-2.4) | 0.7 (0.4-1.3) |
| Recent surgery | 2.3 (1.2-4.2)* | 1.1 (0.6-2.0) |
| Active malignancy | 1.5 (0.9-2.4) | 1.0 (0.6-1.8) |
| Estrogen use | 2.5 (1.3-4.8)* | 1.0 (0.5-2.0) |
| . | SSPE vs PE excluded . | SSPE vs proximal PE . |
|---|---|---|
| OR (95% CI) | ||
| Age >60 y | 1.6 (1.1-2.4)* | 0.9 (0.6-1.4) |
| Male sex | 2.1 (1.4-3.2)* | 0.8 (0.5-1.2) |
| Immobilization | 1.6 (0.9-2.7) | 0.9 (0.5-1.6) |
| Previous VTE | 1.4 (0.8-2.4) | 0.7 (0.4-1.3) |
| Recent surgery | 2.3 (1.2-4.2)* | 1.1 (0.6-2.0) |
| Active malignancy | 1.5 (0.9-2.4) | 1.0 (0.6-1.8) |
| Estrogen use | 2.5 (1.3-4.8)* | 1.0 (0.5-2.0) |
P < .05.
Risk of VTE during follow-up
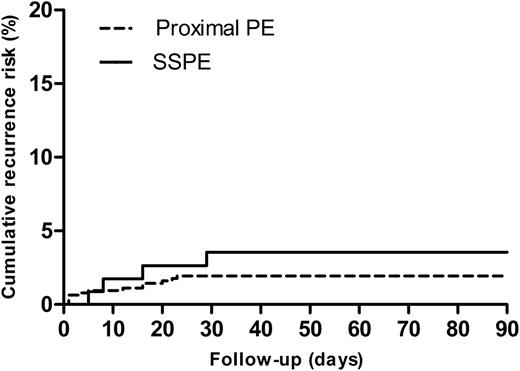
Follow-up was completed in 747 (99.9%) of the patients diagnosed with PE at baseline and in 2974 (99.8%) of the patients in whom PE was ruled out. During 3 months of follow-up, symptomatic recurrent VTE occurred in 4 patients with SSPE (3 patients developed PE, of which 1 case was fatal and 1 patient had DVT) and in 14 patients with segmental or more proximal PE (10 patients developed PE and 4 patients DVT; in 9 patients, PE was adjudicated either as a direct cause of death or PE could not confidently ruled out as cause of death). The respective cumulative risks for recurrent VTE were 3.6% for SSPE and 2.5% for more proximal PE, respectively (Figure 1; P = .42 from the log-rank test). The HR for recurrent VTE was not significantly different for SSPE patients vs patients with more proximal PE (HR: 1.6; 95% CI: 0.5-4.8). Adjustment for age, gender, malignant disease, and history of VTE did not materially influence this HR.
Cumulative recurrence risk SSPE vs proximal PE. Cumulative risk of recurrent VTE for patients with SSPE vs patients with proximal (defined as segmental or central) PE (P = .42 from the log-rank test).
Cumulative recurrence risk SSPE vs proximal PE. Cumulative risk of recurrent VTE for patients with SSPE vs patients with proximal (defined as segmental or central) PE (P = .42 from the log-rank test).
One of the patients with SSPE (25%) and 3 of the patients with more proximal PE (21%) had signs or symptoms suggestive of DVT of baseline.
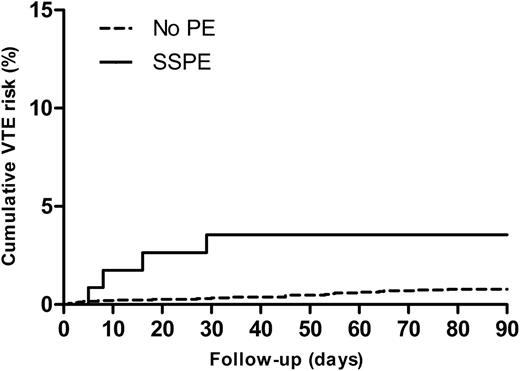
In the group of patients in whom PE was ruled out at baseline, 25 patients (0.8%) developed VTE during follow-up (10 developed DVT, 7 developed PE, and in 8 patients PE was adjudicated either as a direct cause of death or PE could not confidently ruled out as cause of death). The cumulative risk for VTE in this group was 1.1% (Figure 2). The unadjusted HR for the risk of VTE during follow-up for patients with SSPE vs patients with no PE was 4.3 (95% CI: 1.5-12.3). After adjustment for age, gender, malignant disease, and history of VTE, the HR remained statistically significant (3.8 [95% CI: 1.3-11.1]). Malignant disease was independently associated with the occurrence of VTE during follow-up (HR: 3.7; 95% CI: 1.6-8.4).
Cumulative VTE risk SSPE vs no PE. Cumulative risk of VTE during follow-up for patients with SSPE vs patients with no PE (P = .03 from the log-rank test).
Cumulative VTE risk SSPE vs no PE. Cumulative risk of VTE during follow-up for patients with SSPE vs patients with no PE (P = .03 from the log-rank test).
Bleeding complications in patients with PE
Two patients (1.7%) with SSPE and 10 patients (1.6%) with segmental or more proximal PE experienced major bleeding during follow-up. The age- and sex-adjusted OR for major bleeding in patients with SSPE vs those with larger PE was 1.15 (95% CI: 0.25-5.34; P = .86). Two of these bleeding events were adjudicated as fatal; both events occurred in the group of patients with segmental or central PE.
Risk of mortality
Twelve (10.3%) patients diagnosed with SSPE and 40 (6.3%) patients with segmental or central PE died during follow-up. The respective cumulative mortality risks were 10.7% and 6.5% (adjusted HR: 1.5, 95% CI: 0.8-2.8; P = .17 from the log-rank test).
In the patients in whom PE was excluded, 156 (5.2%) patients died during follow-up. Their cumulative mortality risk (5.4%) was significantly lower compared with patients with SSPE (P = .01 from the log-rank test). Multivariate analysis identified malignancy (HR: 5.6; 95% CI: 4.2-7.6), male gender (HR: 1.5; 95% CI: 1.1-2.1), age (HR: 1.04/y; 95% CI: 1.03-1.05), COPD (HR: 1.5; 95% CI: 1.1-2.2), and heart failure (HR: 1.9; 95%: 1.3-2.7) as independent predictors for mortality. After adjustment for these covariates, the HR for mortality was 1.4 (95% CI: 0.8-2.6) for patients with SSPE compared with those in whom PE was ruled out.
Discussion
To our knowledge, the present study is the largest in patients with SSPE and is the first where patients with more proximally located PE as well as patients without PE as reference groups served for comparison. Two important conclusions can be drawn from our findings. First, with regard to the clinical outcome in terms of recurrent VTE, bleeding complications, and mortality, patients with SSPE appear to mimic those with PE localized in more proximal pulmonary arteries. This is supported by the observation of a similar VTE risk profile in both groups. Second, patients with SSPE significantly differ from patients in whom PE was ruled out both in terms of thromboembolic risk profile and incidences of VTE and mortality during follow-up. The latter appeared to be driven by the presence of older age and comorbidities, including malignancy, COPD, and heart failure.
These findings challenge the hypothesis that a diagnosis of SSPE might be clinically insignificant. Evidence for this latter hypothesis was derived from a recent systematic review assessing the rates of SSPE diagnoses on multi-detector and single-detector CTPA examinations.8 Although the proportion of detected SSPE increased from 4.7% to 9.4% for single- compared with multi-detector CTPA, the rate of recurrent VTE in patients in whom PE was ruled out and who were thus left untreated did not differ between the groups (0.9% vs 1.1%). Based on these results, the authors concluded that the additional SSPE cases detected by multi-detector CTPA may be clinically irrelevant. This, however, should be regarded as indirect evidence given that the outcome of patients with SSPE was not directly assessed. More indirect evidence supporting the concept that the increased proportion of SSPE detected by CTPA might be clinically insignificant comes from a large population-based study.17 Based on discharge-level data, Wiener et al17 noticed an increased incidence of PE diagnosis following the introduction of CTPA, whereas the mortality risk remained unchanged and the case-fatality rate decreased. The authors referred to these findings as evidence of overdiagnosis, defined as the detection of an abnormality, specifically small pulmonary emboli, that will never cause symptoms or death. Again, this study does not provide us with direct evidence that the additional PE cases detected by CTPA are harmless. Furthermore, the study does not inform us on the risk of recurrent VTE. Although the decreasing case-fatality rate does suggest that isolated, small pulmonary emboli are less likely to be a direct cause of death, their presence may still reflect a patients’ prothrombotic state and therefore be associated with an increased risk of thrombus extension or VTE recurrence in the future.
If SSPE would represent a distinctive subset of thromboembolic disease or even a physiological finding, we postulated that this would translate to a distinct thromboembolic risk profile and clinical outcome, or that the clinical characteristics of these patients would be more comparable with those of patients without PE. However, we found that both the risk profile and outcome of patients with SSPE largely overlapped with those with more proximal PE, suggesting a similar underlying pathophysiology.
Supporting evidence for our findings comes from the recently published EINSTEIN-PE study18 in which the efficacy and safety of the novel oral anticoagulant rivaroxaban was compared with vitamin K antagonists for the treatment of PE. From that study, separate analyses were performed with respect to the anatomic location of PE. In both treatment arms, similar rates of recurrent VTE were observed for patients with anatomically limited PE (defined as ≤25% of vasculature of a single lobe) vs those with extensive PE (defined as multiple lobes and >25% of entire pulmonary vasculature): 1.6% vs 1.7%, respectively, in the rivaroxaban group and 1.3% vs 1.4% in the standard-treatment group. Although the definition used for anatomically limited PE may also include segmental PE, one would have expected a lower rate of recurrent VTE in these patients in case SSPE would have had no clinical significance. In line with our findings, these data suggest that the risk of recurrent VTE is not influenced by the anatomic location of PE. It seems more likely that persistent risk factors for recurrent VTE are better risk predictors than the location of the PE. A recent population-based study demonstrated that active malignancy is by far the strongest predictor for recurrent VTE.19 Indeed, in the present study, active malignancy was independently associated with the occurrence of VTE during follow-up.
A potential limitation of our study is that an independent radiologist did not confirm the diagnosis of SSPE in the majority of cases. It has recently been demonstrated that significant differences in the interpretation of SSPE among radiologists could occur.20 Although all CTPAs were assessed according to a prespecified protocol, it cannot be ruled out that some of our patients were misclassified as having SSPE; this, however, reflects the diagnostic process of SSPE in daily clinical practice. Second, our definition used for SSPE included both single and multiple SSPE. We were therefore unable to investigate whether the number of emboli and amount of branches affected influence the prognosis of SSPE patients. Third, the absolute incidences of recurrent VTE, bleeding complications, and mortality were small. Although we did not detect a difference in outcome between SSPE patients and those with proximal PE, our study might be underpowered to detect small differences. Our findings should thus be considered hypothesis generating and need to be confirmed in larger studies. Fourth, the presence of DVT at baseline was not systematically assessed; this has recently been identified as an independent predictor for mortality in patients with acute PE.21 However, the proportions of patients who had signs and symptoms suggestive of DVT did not significantly differ between patients with SSPE and those with more proximal PE. Finally, it should be noted that this study was not designed to answer questions about the benefit of anticoagulant treatment in patients with SSPE; all patients included in this analysis were treated. There is a need for prospective studies assessing the outcome and management of SSPE before considering distinct management guidelines for this specific group of PE patients. Indeed, a prospective management study assessing the safety of withholding anticoagulation in patients with isolated symptomatic SSPE, without DVT on bilateral lower extremity compression ultrasonography, is currently being conducted (NCT01455818).
In conclusion, in contrast to the common belief that SSPE represents a benign subset of VTE, this study shows that patients with symptomatic SSPE appear to mimic those with segmental or more proximal PE as regards their risk profile and short-term clinical course. Risk factors for VTE were shown to be associated with SSPE, and the incidences of recurrent VTE and mortality were higher among SSPE patients, compared with those without PE.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.L.d.E. contributed to the study concept and design, analysis and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; J.v.E. contributed to the analysis and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; F.A.K. contributed to the acquisition of data, analysis and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; L.J.K. contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; M.J.H.A.K. contributed to the acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content; P.W.K. contributed to the acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content; H.R.B. contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; and M.V.H. contributed to the study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul den Exter, Department of Thrombosis and Hemostasis, Leiden University Medical Center, Albinusdreef 2, PO Box 9600, 2300 RC, Leiden, The Netherlands; e-mail: p.l.den_exter@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal