Key Points
Scl operates both downstream of Kit to control the survival of Kit+ multipotent and erythroid progenitors and upstream of Kit to determine Kit expression levels.
Scl and Kit establish a positive feedback loop in hematopoietic progenitors.
Abstract
SCL/TAL1, a tissue-specific transcription factor of the basic helix-loop-helix family, and c-Kit, a tyrosine kinase receptor, control hematopoietic stem cell survival and quiescence. Here we report that SCL levels are limiting for the clonal expansion of Kit+ multipotent and erythroid progenitors. In addition, increased SCL expression specifically enhances the sensitivity of these progenitors to steel factor (KIT ligand) without affecting interleukin-3 response, whereas a DNA-binding mutant antagonizes KIT function and induces apoptosis in progenitors. Furthermore, a twofold increase in SCL levels in mice bearing a hypomorphic Kit allele (W41/41) corrects their hematocrits and deficiencies in erythroid progenitor numbers. At the molecular level, we found that SCL and c-Kit signaling control a common gene expression signature, of which 19 genes are associated with apoptosis. Half of those were decreased in purified megakaryocyte/erythroid progenitors (MEPs) from W41/41 mice and rescued by the SCL transgene. We conclude that Scl operates downstream of Kit to support the survival of MEPs. Finally, higher SCL expression upregulates Kit in normal bone marrow cells and increases chimerism after bone marrow transplantation, indicating that Scl is also upstream of Kit. We conclude that Scl and Kit establish a positive feedback loop in multipotent and MEPs.
Introduction
Blood lineage specification from multipotent hematopoietic stem cells (HSCs) is thought to be mainly driven by the differential expression of transcription factors that activate specific genetic programs through bistable behaviors resulting from combinatorial or antagonistic interactions.1 In addition, hematopoietic cells require constant signaling from their environment for survival. How environmental signals are integrated by hematopoietic cells and how survival, differentiation, and growth are coordinated at the molecular level still remain to be clarified. Within the network of transcriptional regulators,1 several factors appear to act as central nodes, possibly by controlling more than 1 cell fate–determining process as discussed subsequently.
Steel factor (SF), referred to as Kit ligand, stem cell factor, or mast cell growth factor, is essential for definitive hematopoiesis in vivo (reviewed by Kent et al2 ). Mutations in the loci coding for SF or for its tyrosine kinase receptor, c-Kit, cause hematopoietic deficiency and anemia.3,4 Hypomorphic Kit alleles impair the development of erythroid progenitors5 and the long-term maintenance of HSCs in vivo.6 Kit suppresses apoptosis in cell lines,7 in primitive progenitors,8 and in HSCs.9 Moreover, depending on the cell type, increased survival rates could be either B-cell lymphoma 2 (Bcl2) dependent or independent,10,11 suggesting that Kit may activate multiple survival pathways.
The Scl/Tal1 (stem cell leukemia) gene encodes a basic helix-loop-helix (bHLH) transcription factor, which is essential for the onset of hematopoiesis. Namely, Scl−/− embryos lack all primitive and definitive hematopoietic lineages.12,13 In the adult, Scl is expressed in multipotent and erythroid progenitors,14,15 as well as in populations endowed with HSC activity.16,17 Accordingly, Scl controls erythoid and megakaryocytic differentiation,18-21 as well as long-term HSC competence under conditions of extensive replicative stress16 but not in conditions approaching steady-state,21,22 possibly due to redundancy with Lyl1.17
A role for SCL in promoting hematopoietic cell survival was first documented in a T-cell line,23 in the primitive erythroid lineage in both murine24 and zebra fish embryos,25 as well as in adult HSCs.17 We previously reported that Scl interacts with Vegf to suppress apoptosis at the onset of hematopoiesis,24 raising the possibility that Scl may also interact with genes encoding growth factors or their receptors in adult hematopoiesis. Indeed, SCL occupies Kit regulatory sequences in primary hematopoietic progenitors,26,27 and Scl levels determine Kit expression levels in the TF-1 cell line.26,28 Conversely, SF sustains Scl expression in primary erythroid cells29 and human CD34+ cells, suggesting that Scl is required for SF antiapoptotic activity.30 Together, these observations suggest that Scl may operate both upstream and downstream of Kit.
Materials and methods
Mice
All mice were housed in a pathogen-free animal facility according to institutional animal care and use guidelines. Transgenic mice expressing the human SCL gene31 and the SclLacZ knock-in mice15 have been backcrossed onto a C57BL/6 background for more than 8 generations. C57BL/6 and W41/41 mice were from The Jackson Laboratories (Bar Harbor, ME). β-Galactosidase activity was analyzed as previously described16 using the Scl-LacZ knock-in mouse model.15
Cell line
The TF-1 human cell line was a gift from Dr T. Kitamura (The Institute of Medical Science, Tokyo, Japan). The cells were maintained in Iscove modified Dulbecco medium supplemented with 10% fetal calf serum and 5 ng/mL human granulocyte macrophage–colony-stimulating factor CSF (GM-CSF). The cells were passaged every second day at 1.5 × 105/mL.
Vectors and gene transfer into bone marrow cells
The MSCV-neor, MSCV-YFP, and MSCV-GFP plasmids containing either human SCL or human ΔbSCL, or asSCL were described previously.28 High-titer helper-free recombinant retroviruses were produced using the GP+E 86 ecotropic virus-packaging cell line.28
The 5-fluorouracil (150 mg/kg) bone marrow cells were prestimulated for 48 hours with 100 ng/mL murine SF, 10 ng/mL human interleukin (IL) 6, 100 ng/mL human IL-11, and 5 ng/mL murine IL-3, at 1 × 106 cells per mL, and cocultured on irradiated (1500 cGy) virus-producing GP+E-86 cells in the presence of polybrene (0.8 μg/mL; Sigma-Aldrich) for 48 hours. In the double-infection strategy, a mixture of GP+E-86 cells producing MSCV-EYFP-SCL and MSCV-EGFP-ΔbSCL retroviruses was seeded for infection. The GP+E-86 producer mixture was calculated such that target cells were exposed to similar viral titers for both viruses.
Flow cytometry analysis, cell sorting, and methyl-cellulose assays
Expression microarray analyses
TF-1 cells expressing either MSCV-GFP or MSCV-GFP-ΔbSCL were stimulated with GM-CSF (5 ng/mL) or SF (20 ng/mL) for 6 hours. RNA was purified using Qiagen RNeasy Mini Kit (P/N 741104) and resuspended in 5 μL of ribonuclease-free water at a concentration of 0.05 μg/μL. Complementary DNA (cDNA) synthesis and hybridization to DNA chip was performed by the Ottawa Genome Centre as follows: cDNA was first labeled using the GeneChip Eukaryotic Small Sample Target Labeling protocol developed by Affymetrix and then hybridized to the probe array HG-U133A (Affymetrix). Normalization and summarization were performed with GeneChip Robust Multichip Analysis (http://www.bioconductor.org), and statistical analysis was performed in R (http://www.r-project.org). Following a false discovery rate multiple test correction,33 genes that show a significant change (q ≤ 0.1) were classified into upregulated by ΔbSCL or downregulated. Genes were subsequently selected based on their role in apoptosis (Gene Expression Omnibus accession number: GSE48557).
Gene expression and chromatin immunoprecipitation
RNA extraction, cDNA preparation, and specific polymerase chain reaction (PCR) amplifications were performed as described.34 Quantitative real-time PCR was performed on MX3000 (Stratagene) or ABI apparatus (ABI) using QuantiTect SYBR Green PCR kit (Qiagen) or TaqMan PCR master mix (ABI). Primer sequences are listed in the supplemental Materials.
Chromatin immunoprecipitation (ChIP) assays were performed essentially as described previously,26 using TF-1 cells, and described in the supplemental Materials.
Statistical analysis
P values were determined with the Student t test using data from at least 3 experiments.
Results
SCL is essential for c-Kit–dependent cell survival
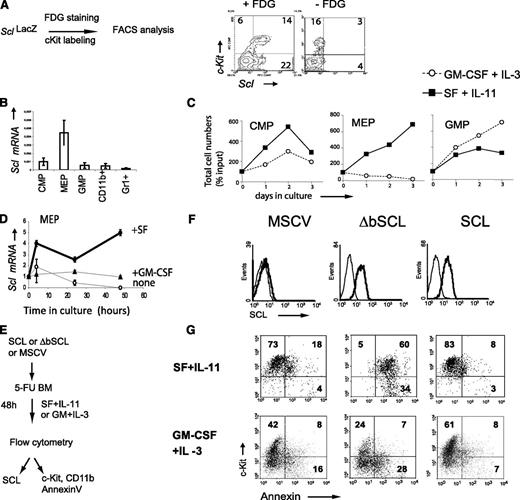
Scl and Kit are coexpressed in a subpopulation of bone marrow cells26 as shown by high β-galactosidase activity in Lin–Kit+ bone marrow cells from SclLacZ mice (+ FDG, Figure 1A) and by reverse transcription (RT)–PCR analysis of purified Kit+ progenitors: multipotent progenitors (common myeloid progenitors [CMPs]), megakaryocyte/erythroid progenitors (MEPs), and granulocyte macrophage progenitors (GMPs) (Figure 1B; supplemental Figure 1A). The lineage potentials of purified progenitors were confirmed in methylcellulose cultures (supplemental Figure 1B). All progenitors survive in response to SF and IL-11, as well as IL-3 and GM-CSF, albeit with significant differences: SF/IL-11 preferentially stimulated CMP and MEP (Figure 1C), whereas GM-CSF/IL-3 was more potent in GMP (Figure 1C). In particular, SF induced a fourfold increase in Scl mRNA levels in Kit+ progenitors when compared with GM-CSF stimulation (Figure 1D), as reported.29,30 Therefore, Scl expression levels are sensitive to c-Kit activity, and SCL/c-Kit coexpression defines progenitor-enriched populations.
SCL is expressed in Kit+cells and controls Kit-dependent cell survival. (A) Hematopoietic progenitors coexpress Scl and Kit. Scl gene transcription was monitored by β-galactosidase staining (+FDG) of bone marrow cells from SclLacZ mice. The substrate was omitted in the negative control (–FDG). (B) Kit+ populations enriched in hematopoietic progenitors, CMP, MEP, and GMP, were purified from wild-type (WT) bone marrows. Scl expression was analyzed by quantitative RT-PCR. Messenger (mRNA) levels were normalized using Hprt as an internal control. The means ± standard deviation (SD) of 3 experiments are shown. (C) These progenitors were maintained in culture with SF and IL-11 or GM-CSF and IL-3. Viable cell counts were monitored at the indicated times. (D) SF but not GM-CSF stimulation of Kit+Sca−Lin− progenitors upregulates Scl mRNA. Purified Kit+Sca-1–Lin– progenitors were stimulated with SF, GM-CSF, or control medium. Scl expression was determined at different time points by quantitative RT-PCR. mRNA levels were normalized using S16 as an internal control and then compared with expression levels at t = 0. (E) Strategy used to test the role of Scl in the survival of Kit+ cells. The 5-fluorouracil (5-FU) mouse bone marrow cells were infected with MSCV-neor retrovirus carrying human SCL, a DNA-binding defective mutant (ΔbSCL), or the control empty vector. (F) Kit+ cells were analyzed for the presence of human SCL by immunofluorescence staining with a monoclonal mouse anti-human SCL (thick line). Staining with the secondary antibody alone (goat anti-mouse) served as negative control (thin line). (G) After retroviral infection, cells were stimulated with either early acting cytokines (SF + IL-11) or myeloid cytokines (GM-CSF + IL-3) for 2 days in selective media (G418) prior to staining with Kit antibodies and Annexin V (20% to 25% infection efficiency). Dead cells were excluded from analysis by propidium iodide staining. The numbers shown represent the percentages of cells within each quadrant. Data shown (A-G) are typical of 2 or 3 independent experiments.
SCL is expressed in Kit+cells and controls Kit-dependent cell survival. (A) Hematopoietic progenitors coexpress Scl and Kit. Scl gene transcription was monitored by β-galactosidase staining (+FDG) of bone marrow cells from SclLacZ mice. The substrate was omitted in the negative control (–FDG). (B) Kit+ populations enriched in hematopoietic progenitors, CMP, MEP, and GMP, were purified from wild-type (WT) bone marrows. Scl expression was analyzed by quantitative RT-PCR. Messenger (mRNA) levels were normalized using Hprt as an internal control. The means ± standard deviation (SD) of 3 experiments are shown. (C) These progenitors were maintained in culture with SF and IL-11 or GM-CSF and IL-3. Viable cell counts were monitored at the indicated times. (D) SF but not GM-CSF stimulation of Kit+Sca−Lin− progenitors upregulates Scl mRNA. Purified Kit+Sca-1–Lin– progenitors were stimulated with SF, GM-CSF, or control medium. Scl expression was determined at different time points by quantitative RT-PCR. mRNA levels were normalized using S16 as an internal control and then compared with expression levels at t = 0. (E) Strategy used to test the role of Scl in the survival of Kit+ cells. The 5-fluorouracil (5-FU) mouse bone marrow cells were infected with MSCV-neor retrovirus carrying human SCL, a DNA-binding defective mutant (ΔbSCL), or the control empty vector. (F) Kit+ cells were analyzed for the presence of human SCL by immunofluorescence staining with a monoclonal mouse anti-human SCL (thick line). Staining with the secondary antibody alone (goat anti-mouse) served as negative control (thin line). (G) After retroviral infection, cells were stimulated with either early acting cytokines (SF + IL-11) or myeloid cytokines (GM-CSF + IL-3) for 2 days in selective media (G418) prior to staining with Kit antibodies and Annexin V (20% to 25% infection efficiency). Dead cells were excluded from analysis by propidium iodide staining. The numbers shown represent the percentages of cells within each quadrant. Data shown (A-G) are typical of 2 or 3 independent experiments.
SCL binds DNA as a heterodimer with E2A. The SCL mutant lacking its basic domain (ΔbSCL) abrogates DNA binding by SCL/E2A heterodimers and exerts a “dominant-negative” effect on the wild-type protein.19,28 To directly address the importance of SCL downstream of c-Kit, we delivered SCL or the dominant-negative ΔbSCL in primary bone marrow cells using the MSCV retroviral vector (Figure 1E). Both SCL and ΔbSCL proteins were efficiently and similarly expressed in bone marrow cells as assessed by flow cytometry analysis (Figure 1F). Immediately after gene delivery, cells were stimulated with either SF and IL-11, which expand and maintain Kit+ cells, or GM-CSF and IL-3, which induce myeloid differentiation (Figure 1G). Surprisingly, the DNA-binding defective mutant ΔbSCL induced massive apoptosis in Kit+ cells stimulated with SF/IL-11 (60%), whereas control cells harboring the empty vector or wild-type SCL survived well under these conditions (18% and 8% apoptosis, respectively) (Figure 1G upper panels). In contrast, apoptosis in Kit+ cells stimulated with GM-CSF/IL-3 was not affected by the various transgenes (7% to 8%) (Figure 1G lower panels). Apoptosis was not due to defective Kit expression because c-Kit levels remain high at this time point. Together with the upregulation of Scl by SF30 (Figure 1D), these functional studies indicate that Scl is specifically required in response to stimulation by SF/IL11.
ΔbSCL induced–apoptosis could be due to a dominant negative effect on endogenous SCL or, alternatively, to the sequestration of another bHLH factor whose function is essential for the survival of hematopoietic progenitors. To discriminate between these possibilities, we showed that a targeted antisense SCL (asSCL) reproduced the effects of ΔbSCL (49% apoptosis) and induced apoptosis in 44% of bone marrow cells (Figure 2A-B). In this experiment, we used GFP as a marker for gene transfer (GFP+YFP–). Furthermore, apoptosis induced by ΔbSCL-GFP or asSCL-GFP was fully rescued by codelivering SCL cloned in the MSCV-YFP vector (GFP+YFP+) to the same levels as control cells expressing SCL alone (0.7% to 4%). We next monitored apoptosis against c-Kit expression. We observed that ΔbSCL or asSCL induced apoptosis in the Kit+ fraction, and this was reproducibly rescued by SCL (Figure 2C-D). These results confirmed the specificity of ΔbSCL and asSCL and indicated a nonredundant antiapoptotic function for SCL downstream of c-Kit.
SCL rescues apoptosis induced a by a dominant negative or an antisense Scl in Kit+hematopoietic progenitors. (A) SCL-YFP and ΔbSCL-GFP or SCL-YFP and asSCL-GFP were codelivered in WT bone marrow cells for 2 days, and apoptosis was monitored 48 hours after infection. (B) Annexin-V positive cells are illustrated in red. Note that red dots are present in the YFP− fractions, exclusively. Numbers in red represent the % of Annexin-V+ cells within each quadrant. (C) SCL-YFP+ and SCL-YFP− fractions were further analyzed for Kit expression and Annexin-V labeling in ΔbSCL- or asSCL-expressing cells. (D) Apoptosis in Kit+ cells. Data shown are the mean ± SD of n independent experiments.
SCL rescues apoptosis induced a by a dominant negative or an antisense Scl in Kit+hematopoietic progenitors. (A) SCL-YFP and ΔbSCL-GFP or SCL-YFP and asSCL-GFP were codelivered in WT bone marrow cells for 2 days, and apoptosis was monitored 48 hours after infection. (B) Annexin-V positive cells are illustrated in red. Note that red dots are present in the YFP− fractions, exclusively. Numbers in red represent the % of Annexin-V+ cells within each quadrant. (C) SCL-YFP+ and SCL-YFP− fractions were further analyzed for Kit expression and Annexin-V labeling in ΔbSCL- or asSCL-expressing cells. (D) Apoptosis in Kit+ cells. Data shown are the mean ± SD of n independent experiments.
SCL determines the clonal expansion of progenitors
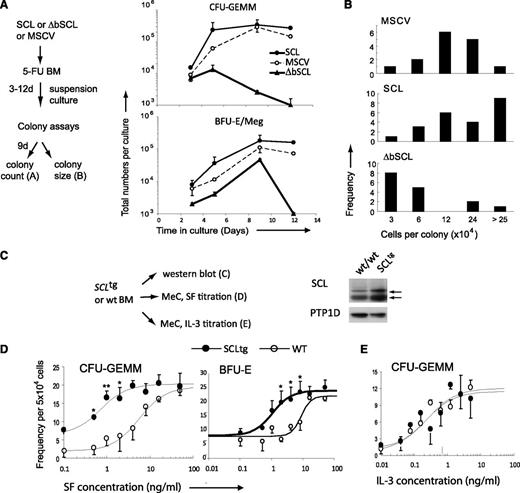
Kit+ cells comprise multipotent progenitors as well as lineage-affiliated progenitors. After retroviral-mediated gene delivery, cells were plated in suspension cultures with either SF/IL11 or IL3/GM-CSF and harvested at various time points to assess apoptosis by flow cytometry (supplemental Figure 1C) as well as the recovery in progenitors by colony assays (Figure 3A). ΔbSCL induced a 20- to 200-fold decrease in multipotent and erythroid/megakaryocyte colony forming cells (CFU-GEMM and BFU-E/Meg) after 5 and 12 days, respectively (Figure 3A), consistent with elevated apoptotic death in cultures with SF/IL11 (supplemental Figure 1C). To assess the capacity for clonal expansion of these multipotent progenitors, the numbers of cells per colony were individually scored (Figure 3B). We found that ΔbSCL-expressing colonies were of smaller size when compared with control colonies (MSCV) or SCL-expressing colonies. Therefore, impeding SCL activity causes apoptosis within the Kit+ Lin– population associated with a massive loss of multipotent progenitors and decreased clonal expansion, suggesting that SCL operates downstream of c-Kit to sustain the survival of multipotent progenitors and control their capacity for clonal expansion.
SCL level determines the output of hematopoietic progenitors. (A) WT bone marrow cells expressing either SCL, ΔbSCL, or control empty vector were plated in suspension culture with SF and IL-11 in selective media (G418). Samples were taken at the indicated times and plated in methylcellulose, and colonies were scored 9 days later. Represented are the cumulative growth curves of multipotent progenitors (CFU-GEMM) (upper panel) or MEPs (BFU-E/Meg) in culture. (B) Bone marrow cells expressing the indicated genes were plated in methylcellulose, and multipotent colonies were aspirated 9 days later, dispersed into single-cell suspensions and counted to determine colony size. Data shown (A-B) are typical of at least 2 independent experiments. (C) Western blot of total protein extracts from unfractionated bone marrow was used to assess SCL protein levels in WT and SCLtg mice. α-Protein tyrosine phosphatase 1D was used as a loading control. (D-E) Bone marrow cells from heterozygous SCLtg mice and WT littermates were cultured in methylcellulose in the presence of increasing concentrations of SF (D) or IL-3 (E). Multipotent colonies (CFU-GEMM) were scored 9 days later. Data represent the mean ± SD of 3 independent experiments performed in duplicate or quadruplicate. Data were analyzed using a nonlinear regression curve fitting routine (ALLFIT). Estimates of the half-effective concentrations of the different ligands on progenitors from SCLtg mice or WT littermates were as follows: 0.7 ± 0.4 (SCLtg) and 6 ± 3 (WT) ng/mL of SF for CFU-GEMMs or BFU-Es, and 0.2 ± 0.2 (SCLtg) and 0.2 ± 0.1 (WT) ng/mL of IL-3. *P < .05; **P < .01.
SCL level determines the output of hematopoietic progenitors. (A) WT bone marrow cells expressing either SCL, ΔbSCL, or control empty vector were plated in suspension culture with SF and IL-11 in selective media (G418). Samples were taken at the indicated times and plated in methylcellulose, and colonies were scored 9 days later. Represented are the cumulative growth curves of multipotent progenitors (CFU-GEMM) (upper panel) or MEPs (BFU-E/Meg) in culture. (B) Bone marrow cells expressing the indicated genes were plated in methylcellulose, and multipotent colonies were aspirated 9 days later, dispersed into single-cell suspensions and counted to determine colony size. Data shown (A-B) are typical of at least 2 independent experiments. (C) Western blot of total protein extracts from unfractionated bone marrow was used to assess SCL protein levels in WT and SCLtg mice. α-Protein tyrosine phosphatase 1D was used as a loading control. (D-E) Bone marrow cells from heterozygous SCLtg mice and WT littermates were cultured in methylcellulose in the presence of increasing concentrations of SF (D) or IL-3 (E). Multipotent colonies (CFU-GEMM) were scored 9 days later. Data represent the mean ± SD of 3 independent experiments performed in duplicate or quadruplicate. Data were analyzed using a nonlinear regression curve fitting routine (ALLFIT). Estimates of the half-effective concentrations of the different ligands on progenitors from SCLtg mice or WT littermates were as follows: 0.7 ± 0.4 (SCLtg) and 6 ± 3 (WT) ng/mL of SF for CFU-GEMMs or BFU-Es, and 0.2 ± 0.2 (SCLtg) and 0.2 ± 0.1 (WT) ng/mL of IL-3. *P < .05; **P < .01.
SCL sets threshold for the response of multipotent progenitors to SF
We next addressed the question of whether SCL levels determine the threshold of response to SF. We took advantage of the SCL transgenic mouse model in which the expression of the human SCL gene is driven by the ubiquitous SIL promoter,31 resulting in a twofold increase in SCL protein levels when compared with age-matched controls (Figure 3C). Human and mouse SCL proteins are identical in their bHLH regions, and the human gene induces T-cell acute lymphoblastic leukemia in mice.31 We compared SF dose-response curves between WT and SCLtg mice. Multipotent (CFU-GEMM) and erythroid (BFU-E) (Figure 3D), but not granulocyte/macrophage colony forming cells (CFU-GM) (supplemental Figure 2A), from SCL transgenic mice were reproducibly 10-fold more sensitive than wild-type cells to SF stimulation. In contrast, the SCL transgene did not affect dose-response curves for IL-3 (Figure 3E) and GM-CSF (supplemental Figure 2B-C), nor the level of c-Kit expression in bone marrow cells (supplemental Figure 3). Together, our observations indicate that SCL levels set thresholds for the response of multipotent and erythroid progenitors to SF without affecting their response to IL3 or GM-CSF.
SCL controls the expression of survival genes
We next set out to identify genes whose expression is modulated by SF stimulation and mediated by SCL, using the human TF-1 cell line for global gene expression analysis, followed by an assessment of differential gene expression in purified progenitors from W41/41 mice that are genetically deficient for c-Kit signaling.
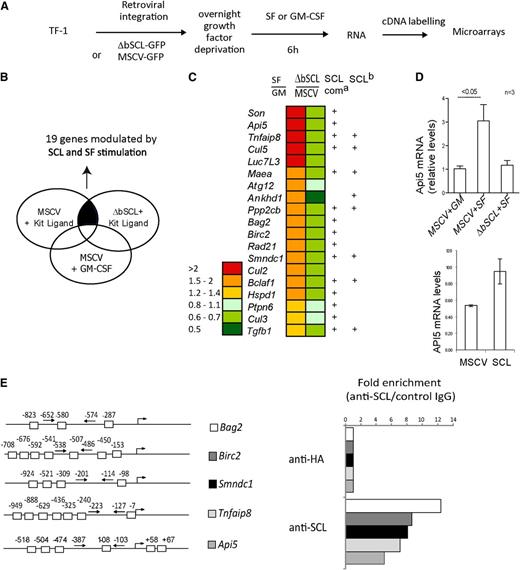
We capitalized on the fact that cells maintained with GM-CSF can tolerate ΔbSCL expression to perform retroviral infection and G418 selection in GM-CSF–containing cultures. Following gene transfer, control cells (MSCV-GFP) or cells expressing ΔbSCL were deprived of growth factor overnight. Cells were then stimulated with either GM-CSF or SF for 6 hours, a time point at which ΔbSCL-expressing cells were still viable. Furthermore, this early time point is more likely to reveal direct SCL targets. Gene expression was analyzed by hybridization to the Affymetrix probe array HG-U133A (Figure 4A). c-Kit–regulated genes were extrapolated from gene expression profiles of control cells (empty vector) stimulated with SF or GM-CSF. Secondly, we searched for SCL-regulated genes by selecting those that were differentially expressed in ΔbSCL cells vs control cells (MSCV), both under SF stimulation. Comparison of genes obtained in both analyses revealed that 19 genes associated with cell survival were comodulated by c-Kit signaling and by SCL function (Figure 4B-C). Of these, 5 genes are antiapoptotic, Api5, Birc2, Cul5, Tnfaip8, and Son; 2 others have dual pro- and antiapoptotic activities, Tgfb1 and Hspd1; and 3 are reported to be proapoptotic, Bag2, Bclaf1, and Smndc1. In addition, 4 genes are linked to the cell cycle, Cul2, Cul3, Cul5, and Rad21; 1 to autophagy, Atg12; and 1 to anchorage-independent growth and tumorigenesis, Ankhd1. Remarkably, 15 of these 19 genes were mapped to ChIP-Seq peaks reported by Wilson et al associated with SCL, LMO2, and/or GATA-2 in a murine hematopoietic progenitor cell line,35 and 7 of those were independently reported by Kassouf et al in primary erythroid fetal liver cells27 (Figure 4C; supplemental Figure 4A). Furthermore, these ChIP-Seq peaks mapped mostly to the promoter regions (86%) and to a lesser extent to exons (46%) and introns (50%) of our gene set, but very rarely to 3′ regions (7%, supplemental Figure 4A). Together, this concordance with ChIP-Seq peaks strongly supports the view that many of the genes shown in Figure 4C are direct targets of regulation by the SCL complex.
SCL regulates the expression of survival genes in progenitors. (A) Strategy used to identify genes controlled by SCL and Kit in human CD34+ TF-1 cells. (B) Schematic diagram representing our approach to analyze microarray data. We performed a paired comparison between TF-1 cells stimulated with either SF or GM-CSF and TF-1 cells harboring either the empty vector or the ΔbSCL under SF treatment. Nineteen survival genes were found to be coregulated by SCL and Kit signaling. (C) Relative expression levels for the 19 genes modulated by SCL and Kit. Ratio of gene expression levels of control cells (MSCV) stimulated with SF over GM-CSF, and of ΔbSCL-expressing cells over control cells, both stimulated with SF. a, ChIP-Seq peaks identified by Wilson et al35 with anti-SCL, LMO2, or GATA-1 antibodies; b, ChIP-Seq peaks identified by Kassouf et al27 with anti-SCL. (D) TF-1 cells harboring either the empty vector or ΔbSCL were treated with GM-CSF or SF (upper panel), or TF-1 cells expressing SCL were treated with GM-CSF (lower panel). Api-5 mRNA levels were assessed by semiquantitative RT-PCR. Data shown are the means ± SD of n experiments. (E) SCL occupies several loci by ChIP. E boxes within the proximal promoters of the indicated genes are shown (left panel). TF-1 cell chromatin extracts were subjected to immunoprecipitation with SCL antibody or with species-matched control immunoglobulin G (right panel). Fold enrichments were calculated as described in “Materials and methods.”
SCL regulates the expression of survival genes in progenitors. (A) Strategy used to identify genes controlled by SCL and Kit in human CD34+ TF-1 cells. (B) Schematic diagram representing our approach to analyze microarray data. We performed a paired comparison between TF-1 cells stimulated with either SF or GM-CSF and TF-1 cells harboring either the empty vector or the ΔbSCL under SF treatment. Nineteen survival genes were found to be coregulated by SCL and Kit signaling. (C) Relative expression levels for the 19 genes modulated by SCL and Kit. Ratio of gene expression levels of control cells (MSCV) stimulated with SF over GM-CSF, and of ΔbSCL-expressing cells over control cells, both stimulated with SF. a, ChIP-Seq peaks identified by Wilson et al35 with anti-SCL, LMO2, or GATA-1 antibodies; b, ChIP-Seq peaks identified by Kassouf et al27 with anti-SCL. (D) TF-1 cells harboring either the empty vector or ΔbSCL were treated with GM-CSF or SF (upper panel), or TF-1 cells expressing SCL were treated with GM-CSF (lower panel). Api-5 mRNA levels were assessed by semiquantitative RT-PCR. Data shown are the means ± SD of n experiments. (E) SCL occupies several loci by ChIP. E boxes within the proximal promoters of the indicated genes are shown (left panel). TF-1 cell chromatin extracts were subjected to immunoprecipitation with SCL antibody or with species-matched control immunoglobulin G (right panel). Fold enrichments were calculated as described in “Materials and methods.”
We confirmed by quantitative RT-PCR that Api5 was regulated by SF and SCL in TF-1 cells because c-Kit–induced Api5 mRNA expression was specifically inhibited by the dominant negative ΔbSCL (Figure 4D upper panel). In contrast, overexpression of SCL increased Api5 expression even in the absence of SF stimulation (Figure 4D lower panel). Moreover, we examined the promoter regions of 5 genes, Api5, Bag2, Birc2, Smndc1, and Tnfaip8, and found the presence of multiple E boxes. ChIP using a monoclonal anti-SCL antibody indicated that SCL specifically occupies these promoter regions (Figure 4E) but not Api-5 3′ sequences or Hprt promoter sequences (supplemental Figure 4B). These genes are therefore direct SCL targets in TF-1 cells.
Rescue of the W41 allele by the SCL transgene in vivo
W41/41 mice have a mild macrocytic anemia36 caused by a mutation in the kinase domain resulting in decreased c-Kit catalytic activity.37 We found that Scl levels were twofold decreased in Kit+ progenitors from W41/41 mice compared with wild-type controls (Figure 5A), consistent with an upregulation of Scl by c-Kit activity. To determine whether a twofold increase in SCL could rescue the hematopoietic defects caused by the W41 hypomorphic allele (Figure 5B), we crossed W41/41 mice with SCLtg mice and compared the hematopoietic parameters of W41/41SCLtg mice with those of littermate controls. The moderate level of SCL expression directed by the transgenic cassette did not affect most hematopoietic parameters in a Kit wild-type background, except for a modest 1.5-fold increase in GMPs. W41/41 mice showed decreased hematocrits, which was rescued by the SCL transgene to levels that were comparable to wild type (Figure 5C). Reticulocyte frequencies were not significantly different among the various genotypes (2% on average). Consistent with a mild macrocytic anemia,36 erythrocytes from W41/41 mice but not other genotypes were infrequently larger than normal (Figure 5D; supplemental Figure 5A).
Genetic interaction between Kit and SCL. (A) Scl mRNA levels in purified MEPs from WT and W41/41 mice. (B) Strategy used to test the genetic interaction between Scl and Kit. (C) The SCL transgene rescues the hematocrit of W41/41 mice. Blood from 8- to 12-week-old mice was analyzed, and the hematocrits of individual mice and the mean of each group are illustrated. W41/41 mice exhibit decreased hematocrits compared with age-matched controls (P < .05), which were corrected by the SCL transgene (P = .05). (D) Blood smears from 8- to 12-week-old mice. L, lymphocyte; Plt, platelets R, polychromatophilic erythrocyte (reticulocyte). Two hundred to 300 cells on 2 different slides were scored per mouse (n = 6 for W41/41, and n = 3 for the other genotypes). (E) Analysis of progenitor-enriched populations by flow cytometry. The absolute numbers of progenitors per mice are shown as box plots for n mice of each genotype. *P < .05 compared with wt controls. (F) Bone marrow cells from wt, W41/41, W41/41-SCLtg, and SCLtg mice were cultured in methyl-cellulose, and the frequency of early (BFU-E) and late erythroid progenitors (CFU-E) as well as multipotent (CFU-GEMM) and myeloid progenitors (CFU-GM) was determined. Cultures were performed in triplicate, and data are shown as box plots with the medians as well as the 2 extreme values of each distribution. For CFU-GEMM, CFU-E, and BFU-E, the differences between wt and W41/41, and between W41/41 and W41/41-SCLtg were significant. *P < .05. In contrast, the number of colonies obtained from W41/41-SCLtg and WT mice was not significantly different (P > .1). (G) Gene expression analysis for MEPs from wt, W41/41, W41/41-SCLtg, and SCLtg mice. Expression levels of genes identified in Figure 4C were determined by quantitative RT-PCR. mRNA levels were normalized using Hprt as an internal control, and the expression levels in wt MEPs were set as 1. Shown are the averages ± SD of 2 experiments performed in triplicate (# not determined).
Genetic interaction between Kit and SCL. (A) Scl mRNA levels in purified MEPs from WT and W41/41 mice. (B) Strategy used to test the genetic interaction between Scl and Kit. (C) The SCL transgene rescues the hematocrit of W41/41 mice. Blood from 8- to 12-week-old mice was analyzed, and the hematocrits of individual mice and the mean of each group are illustrated. W41/41 mice exhibit decreased hematocrits compared with age-matched controls (P < .05), which were corrected by the SCL transgene (P = .05). (D) Blood smears from 8- to 12-week-old mice. L, lymphocyte; Plt, platelets R, polychromatophilic erythrocyte (reticulocyte). Two hundred to 300 cells on 2 different slides were scored per mouse (n = 6 for W41/41, and n = 3 for the other genotypes). (E) Analysis of progenitor-enriched populations by flow cytometry. The absolute numbers of progenitors per mice are shown as box plots for n mice of each genotype. *P < .05 compared with wt controls. (F) Bone marrow cells from wt, W41/41, W41/41-SCLtg, and SCLtg mice were cultured in methyl-cellulose, and the frequency of early (BFU-E) and late erythroid progenitors (CFU-E) as well as multipotent (CFU-GEMM) and myeloid progenitors (CFU-GM) was determined. Cultures were performed in triplicate, and data are shown as box plots with the medians as well as the 2 extreme values of each distribution. For CFU-GEMM, CFU-E, and BFU-E, the differences between wt and W41/41, and between W41/41 and W41/41-SCLtg were significant. *P < .05. In contrast, the number of colonies obtained from W41/41-SCLtg and WT mice was not significantly different (P > .1). (G) Gene expression analysis for MEPs from wt, W41/41, W41/41-SCLtg, and SCLtg mice. Expression levels of genes identified in Figure 4C were determined by quantitative RT-PCR. mRNA levels were normalized using Hprt as an internal control, and the expression levels in wt MEPs were set as 1. Shown are the averages ± SD of 2 experiments performed in triplicate (# not determined).
The W41 mutation caused a reduction in bone marrow late erythroid progenitors5 but not myeloid progenitors.5,38 Accordingly, multipotent (CFU-GEMMs) and erythroid (BFU-Es and CFU-Es), but not myeloid (CFU-GMs), colony-forming cells were reproducibly twofold decreased in W41/41 mice when compared with age-matched WT mice (Figure 5F). The frequencies of these progenitors were rescued by the SCL transgene to normal levels (Figure 5F). Finally, the SCL transgene in a wild-type background did not significantly affect the frequency of these progenitors (Figure 3D-E; supplemental Figure 5). Similarly, MEPs and CMPs analyzed by flow cytometry were also significantly decreased in W41/41 mice (P < .0001), but not in W41/41SCL transgenic mice (P = .084). The lack of effect of the SCL transgene on these parameters in a wild-type background led us to conclude a genetic interaction between W41 and SCL. Despite the fact that the SCL transgene caused a modest increase in GMP numbers, GMPs were not affected by the W mutation, suggesting that Scl and cKit do not operate in the same pathway in GMPs or, alternatively, that additional cytokines control GMP numbers in vivo. This increase in GMPs correlates with data published by Dey et al39 indicating that SCL causes an expansion of monocytes. Finally, the Kit+Sca+Lin− population was neither affected by the W41 mutation nor by the SCL transgene (supplemental Figure 5B), as reported.6,16,17 Therefore, a twofold increase in SCL expression is sufficient to correct the defects in hematopoietic progenitors caused by a hypomorphic Kit allele without affecting wild-type mice, further supporting the view that Kit and SCL operate in the same pathway.
To address the question of whether the gene set identified in Figure 4C is subject to regulation by endogenous c-Kit activity and by the SCL transgene in primary hematopoietic progenitors, we purified MEP from wt, W41/41, and W41/41SCLtg mice and assessed the expression levels of this gene set by quantitative RT-PCR (Figure 5G). We observed that 9 genes were significantly downregulated in W41/41 mice when compared with wt MEP (Figure 5G). Moreover, when the SCL transgene was introduced in the W41/41 background, expression levels were rescued to wt levels for 7 genes, whereas there was a partial rescue for the remaining 2 genes. These findings indicate that SCL controls the expression of survival genes in primary progenitors downstream of c-Kit.
Taken together, our functional approaches indicate that Kit and SCL operate within the same genetic pathway to determine the survival and clonal expansion of multipotent and erythroid progenitors.
High SCL levels upregulate Kit expression and enhance bone marrow reconstitution
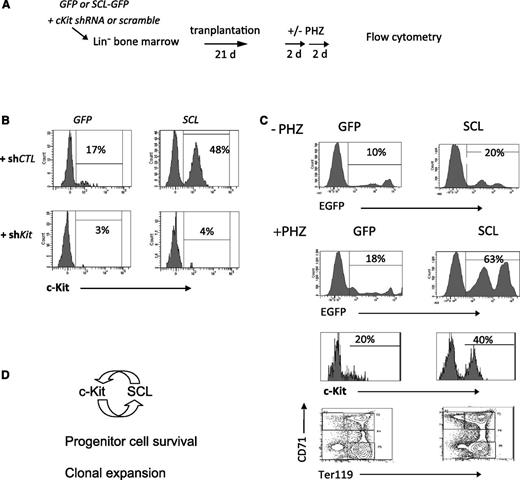
We have previously shown that the SCL transcriptional complex occupies the Kit locus in TF-1 cells and activates Kit promoter activity,26 indicating that Scl is also upstream of Kit. In Scl-proficient cells, the SCL transgene did not further upregulate c-Kit levels. To assess whether higher SCL levels would affect c-Kit levels and modify hematopoietic progenitors, we have designed a 2-step gene delivery. First, SCL (GFP) was overexpressed in Lin-negative bone marrow cells by retroviral-mediated gene delivery for 48 hours, followed by lentiviral delivery of Kit-directed short hairpin RNA (shRNA) (shKit) for 48 hours and puromycin selection for 24 hours, prior to transplantation (Figure 6A). The retroviral gene delivery for SCL allows for higher SCL protein levels (Figure 1F) than transgenic expression (Figure 3C). Transplanted mice were analyzed 25 days later for short-term reconstitution (Figure 6B-C) and stress erythropoiesis (Figure 6C; supplemental Figure 6).
Higher SCL levels upregulate Kit expression. (A) Bone marrow cells were infected with SCL-GFP or the empty GFP vector for 48 hours. Kit shRNA or a control scramble sequence was then delivered by lentiviruses for 24 hours. After puromycin selection for 48 hours, cells were then transplanted into irradiated hosts. Three weeks later, mice were challenged with 2 doses of phenylhydrazine (PHZ) over 4 days. (B) Bone marrow cells were analyzed for c-Kit expression, within the GFP+Lin– fraction. (C) Donor-derived reconstitution (GFP) in control mice (–PHZ) and PHZ-treated mice (+PHZ). Kit+Lin– cells and erythroid differentiation (TER119/CD71) were analyzed in the GFP+ fraction of PHZ-treated mice. (D) Model of a positive feedback loop between c-Kit and SCL. Ligand-activated c-Kit upregulates Scl expression. Higher SCL levels further activate Kit transcription and increase bone marrow reconstitution.
Higher SCL levels upregulate Kit expression. (A) Bone marrow cells were infected with SCL-GFP or the empty GFP vector for 48 hours. Kit shRNA or a control scramble sequence was then delivered by lentiviruses for 24 hours. After puromycin selection for 48 hours, cells were then transplanted into irradiated hosts. Three weeks later, mice were challenged with 2 doses of phenylhydrazine (PHZ) over 4 days. (B) Bone marrow cells were analyzed for c-Kit expression, within the GFP+Lin– fraction. (C) Donor-derived reconstitution (GFP) in control mice (–PHZ) and PHZ-treated mice (+PHZ). Kit+Lin– cells and erythroid differentiation (TER119/CD71) were analyzed in the GFP+ fraction of PHZ-treated mice. (D) Model of a positive feedback loop between c-Kit and SCL. Ligand-activated c-Kit upregulates Scl expression. Higher SCL levels further activate Kit transcription and increase bone marrow reconstitution.
As shown in Figure 6B, shKit’s efficiently decreased c-Kit levels in GFP+ bone marrow cells compared with control scramble sequences (shCTL). SCL upregulated c-Kit expression in Lin-negative cells, and this induction was disrupted by shKit. These results indicate that Kit expression is downstream of SCL as previously reported.26 Bone marrow chimerism assessed by GFP was increased from an average of 10% in control groups (GFP) to 20% by SCL (Figure 6C, upper panel). We next tested the stress response to acute hemolysis. Treatment with phenylhydrazine (+PHZ) increased chimerism in both groups, and the highest levels were observed in the SCL group (63%, Figure 6C, lower panel). The GFP+ population in PHZ-treated mice comprised 80% Ter119+ cells in both groups (data not shown). Therefore, hemolytic stress induced an overall twofold increase in erythroid cells in the bone marrow of control mice and threefold in the SCL group. In adult mice, erythropoiesis shifts from the bone marrow to the spleen in stress response. Hence, PHZ induced a 10- to 50-fold increase in splenic erythroblasts (Ter119+CD71+) and mature erythroid cells (Ter119+CD71–), and this process is not affected by SCL (supplemental Figure 6C). Nonetheless, ectopic SCL expression increased the number of multipotent progenitors (CFU-GEMM) in the spleen (supplemental Figure 6C) in response to erythropoietic stress.
Bone marrow reconstitution in transplantation assays2 as well as PHZ-induced stress response40 are both Kit-dependent. Kit-directed RNA interference decreased bone marrow chimerism from 10% to 18% (Figure 6C) to 1% to 2% regardless of the codelivery of SCL (supplemental Figure 6B). We conclude that SCL increases short-term engraftment, and this is Kit dependent. Therefore, Scl is upstream of Kit in this context. Finally, splenic stress response to acute hemolysis was blunted by Kit RNA interference in all groups as assessed by colony assays and by flow cytometry analysis (supplemental Figure 6C), confirming the importance of c-Kit both in bone marrow recontitution and erythropoietic stress.
Discussion
In the present study, we show that SCL levels are limiting for the clonal expansion of multipotent and erythroid progenitors, that Scl gene dosage specifically determines the threshold of cellular response to SF, and that SCL has the capacity to rescue the hematopoietic deficiency of W41/41 mice. Finally, retroviral delivery of SCL further upregulates c-Kit levels in normal bone marrow cells and enhances marrow reconstitution post transplantation.
SCL and cell survival
Hematopoietic cells require signaling from the environment for their survival, as illustrated by the hematopoietic deficiency of W and Sl mutant mice3,4 caused by defective c-Kit signaling. SF has been shown to suppress apoptosis7 and to act in synergy with other growth factors to promote hematopoietic cell proliferation in vitro.41 In HSCs, however, SF sustains cell survival and quiescence,42 much in the same way as SCL.16 We now provide evidence that Kit and Scl operate within the same genetic pathway to promote hematopoietic cell survival. Furthermore, our observations revealed a unique biological specificity of SCL in directing the response of multipotent progenitors to SF/IL-11 but not to myeloid cytokines (GM-CSF and IL-3). This biological specificity was not observed in cell lines (ie, 32D myeloid and Jurkat lymphoid cell lines).23,43
Despite its essential role at the onset of hematopoiesis, Scl deletion in adult HSCs is innocuous,21 and Scl expressivity in these cells was later revealed by Lyl1 deficiency, which, together with Scl-deficiency, caused apoptosis in Sca+ cells. These observations concur with an antiapoptotic role for both LYL1 and SCL. We have not addressed the importance of LYL1 in the present study. Nonetheless, apoptosis observed here was mostly due to an acute disruption of SCL function or SCL levels because an antisense SCL mirrors the activity of ΔbSCL and both can be rescued by SCL. Interestingly, analysis of ChIP-Seq data from Wilson et al indicates that LYL1 binding overlaps with SCL on 2 of the 15 genes identified here, Tnfaip8 and Tgb1, whereas the Cul2 locus is associated with LYL1 but not the SCL complex.35 Hence, this rather low cooccupancy of LYL1 and SCL on this set of target genes suggests that SCL has both LYL1-redundant and nonredundant activities. The importance of SCL in the survival of Kit+ progenitors observed here is in agreement with the differing contributions of SCL and LYL1 at various developmental stages. SCL is redundant21 with LYL1 in steady-state HSCs17,20,21 but not in the long-term maintenance of HSCs following extensive proliferation.16 Both Lyl144 and Scl22 control the number of CFUS12, whereas Scl but not Lyl1 is required for erythropoiesis and megakaryopoiesis.20,21 In stress erythropoiesis, however, Lyl-1 deficiency45 enhances PHZ response, suggesting that Lyl-1 functions in opposition to Scl in the latter situation.
Genetic models of Scl loss of function (SclLacZ, Sclfl) or gain of function in the mouse or in zebra fish indicate an antiapoptotic role for SCL in hemangioblasts and primitive erythroid cells,24,25 in adult HSCs,17 in erythropoietin-deprived erythroid progenitors,46 and in the survival of leukemic T cells.23,47,48 In contrast, the survival of late erythroid cells20 and of monocytes39 does not depend on SCL function, indicating that SCL may have different functions in progenitors and mature cells.
We have previously shown that Kit is a target of transcription activation by SCL.26,28 Interestingly, Kit activation, as well as the rescue of hematopoiesis during embryonic development,49 does not depend on the capacity of SCL to bind DNA, due to its capacity to recruit other DNA binding factors such as E2A and LMO2-GATA1/2. More recently, SCL is reported to sustain the survival of adult HSCs17 together with LYL1. Here we show that the DNA-binding domain of SCL is essential to fulfill this role, as ectopic expression of ΔbSCL clearly induced apoptosis in these cells, thereby explaining the inability of DNA-binding defective SCL mutant to rescue definitive hematopoiesis.50 We have expressed human SCL in mouse bone marrow cells because human and mouse SCL proteins are identical in their bHLH domains and more than 90% identical in their Nt domains.51 Strikingly, the basic domain is conserved in Drosophila with only 2 conservative changes, I190V and S195T. This phylogenetic conservation underscores the importance of the basic domain for SCL function.
At the molecular level, we found that SCL and c-Kit control a common survival gene signature. Strikingly, 79% of the genes identified here in the human TF-1 cell line were recently reported to be direct targets of the SCL complex by ChIP-Seq in HP7, a murine multipotent embryonic stem cell–derived cell line,35 and 42% in murine fetal liver cells.27 In particular, there was almost complete overlap between the 2 sets as 7 of 8 genes in the second set27 were also found in the first set.35 Moreover, all of the genes identified using the human TF-1 model cell line were expressed in purified murine MEP, half of which were affected by the W41 mutation and rescued by the SCL transgene. This remarkable conservation of gene sets across species and across several hematopoietic cell types obtained with different technologies strongly suggests that the gene sets that we have identified here are likely to be functionally important direct SCL targets.
Genetic interaction between Scl and Kit
Interestingly, the reported phenotype of Scl deficiency in the adult20-22 mirrors that of W41/41 mice bearing a hypomorphic Kit allele. In both cases, myeloid progenitors are unaffected, whereas erythroid progenitors are impaired.38 Furthermore, SCL is required for proper erythroblast maturation.21 The W41 mutation causes macrocytic anemia due to the V831M mutation in the kinase domain that decreases c-Kit catalytic activity.37 The phenotypic concordance of the W41 mutation and the conditional Scl knockout in adult mice suggests that the 2 genes may operate within the same pathway. Here, we show that a twofold increase in SCL levels did not further increase c-Kit levels in hematopoietic cells but was sufficient to increase the sensitivity of c-Kit–proficient multipotent/erythroid progenitors to SF and to rescue a moderate Kit deficiency in W41/41 mice by restoring their hematocrits, MEP numbers, as well as gene expression in MEPs. These results indicate a physiological role for SCL downstream of c-Kit during steady-state hematopoiesis to secure the survival of Kit+ MEPs.
Retroviral delivery of SCL induces high levels of SCL expression in murine bone marrow cells, upregulates c-Kit expression, and favors marrow reconstitution, which differs from transgenic SCL expression discussed previously. Thus, SCL acts upstream of Kit under these conditions. Furthermore, SCL-enhanced bone marrow reconstitution is Kit dependent. Together with the rescue of hematopoietic parameters in W41/41 mice, our observations indicate that SCL expressivity in multipotent and erythroid progenitors requires a minimum threshold of Kit activity afforded by KitV831M. In HSCs and progenitors, c-Kit triggers multiple signaling pathways that include AKT, S6 kinase, c-Jun N-terminal kinase,40 extracellular signal-regulated kinase 1/2, and Janus kinase–signal transducer and activator of transcription (reviewed by Kent et al2 ). Therefore, SCL overexpression is likely to rescue only pathways that converge on SCL-dependent transcriptional regulation. It is possible that KitV831-dependent pathways control Scl mRNA levels in MEPs.
Signaling from the environment to hematopoietic transcription factors: positive feedback loops
Regulatory interactions within a network of lineage-affiliated transcription factors are believed to maintain multipotent progenitors/stem cells in an undifferentiated state. In such systems, perturbations that lock into place a “tip-over” metastable state could initiate the process of lineage determination. SCL is an upstream activator of Kit transcription,26,27,35 and based on data presented here, SCL is also required downstream of Kit (Figure 6D). Both SCL and Kit shape hematopoietic cell fate by sustaining HSC survival, quiescence, and long-term maintenance.16,17,42 We therefore propose that Scl and Kit are part of a positive feedback loop that consolidates the generation of HSCs during development and favors the megakaryocyte/erythroid lineage choice in multipotent progenitors.
Critical cellular and developmental transitions are driven by positive feedback loops. Start, a point of irreversible commitment into the cell cycle in yeast, is controlled by a positive feedback loop in which the Cyclin1/2-cyclin-dependent kinase 1 complex favors Cyclin1/2 transcription by MBF and SBF transcription factor complexes (reviewed by Ferrell52 ). Another example of a positive feedback loop is the early B-cell factor and myogenic differentiation transcriptional regulators that maintain myogenic differentiation expression and stabilize the muscle cell fate in Xenopus.53 A similar positive feedback loop may exist in the granulocytic lineage. Granulocyte colony-stimulating factor (G-CSF) signaling regulates CCAAT/enhancer binding protein (C/EBP) ε54 and favors granulocytic differentiation at the expense of macrophages. In addition, the gene encoding the G-CSF receptor is a target of C/EBPα55 and C/EBPε,56 implying the existence of a positive feedback loop between the G-CSF receptor and C/EBPα-C/EBPε. We propose that lineage output in the hematopoietic system is determined by positive feedback loops established by critical transcription factors and/or ligand/receptor pairs.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE48557).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Danièle Mathieu-Mahul (Institut National de la Santé et de la Recherche Médicale, Marseille, France) for the anti-SCL antibody; Drs Lorraine Robb and Glenn Begley (Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC, Australia) for the SclLacZ mouse; Dr Sabine Herblot, Nathalie Tessier, Éric Massicotte, and Danièle Gagné for assistance with flow cytometry; Véronique Mercille and Véronique Litalien for mouse husbandry; André Haman and Marianne St.-Denis for help with colony assays; Christian Charbonneau for image acquisition; and Dr Pierre Chagnon and Raphaëlle Lambert for quantitative RT-PCR.
This work was funded by grants from the Canadian Cancer Society Research Institute (M.T.), the Canadian Institutes for Health Research, the Leukemia Lymphoma Society of Canada and the Cancer Research Society Inc. (T.H.), the Intramural Research Program of the US National Institutes of Health National Cancer Institute (P.D.A.), studentships from the Fonds FCAR (J.L.) and from the Faculty of Graduate Studies of the Université de Montréal (J.L.), the National Science and Engineering Research Council (R.M.), and the Cole Foundation (M.T. and B.G.). The infrastructure of the Institute for Research in Immunology and Cancer is supported in part by a group grant from the Fonds de Recherche du Québec en Santé, the Canada Foundation for Innovation and the Networks of Centres of Excellence through the Centre of Excellence for Commercialization and Research program.
Authorship
Contribution: J.L., G.K., M.T., B.G., and R.M. performed experiments, analyzed the data, and made the figures; S.L. contributed to the statistics and bioinformatic analyses; and J.L., G.K., M.T., P.D.A., and T.H. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.L. is Clinical Research Institute of Montreal, Montréal, QC, Canada.
Correspondence: Trang Hoang, Institute for Research in Immunology and Cancer (IRIC)–University of Montréal, P.O. Box 6128, Downtown Station, Montréal, QC, Canada H3C 2J7; e-mail: trang.hoang@umontreal.ca.
References
Author notes
G.K., M.T., and B.G. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal