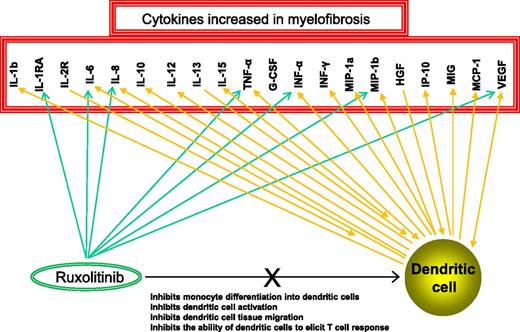
In this issue of Blood, Heine et al demonstrate the dose-dependent inhibitory effect of ruxolitinib on the generation of dendritic cells (DCs) from monocytes. The drug also inhibited DC activation, tissue migration, and induction of allogeneic or antigen-specific T-cell responses, including in vivo viral clearance.1
Cytokines that are aberrantly expressed in MF are known to be developmentally required or secreted by DCs and also targeted by ruxolitinib. G-CSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; INF, interferon; IP-10, interferon-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MIG, monokine induced by interferon-gamma; MIP-1a, macrophage inflammatory protein 1a; VEGF, vascular endothelial growth factor.
Cytokines that are aberrantly expressed in MF are known to be developmentally required or secreted by DCs and also targeted by ruxolitinib. G-CSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; INF, interferon; IP-10, interferon-inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MIG, monokine induced by interferon-gamma; MIP-1a, macrophage inflammatory protein 1a; VEGF, vascular endothelial growth factor.
Human DCs originate from hematopoietic stem cells that give rise to developmentally and functionally distinct DC subsets with the ability to infiltrate lymphatic and other tissues, process and present antigens, and effectively prime T cells toward effector or regulatory responses.2 Accordingly, DCs can augment or dampen host inflammatory response, and their inhibition might result in increased propensity to infections, compromised immune response to cancer, and dysregulated autoimmunity.2
Growth factors (eg, granulocyte-macrophage colony-stimulating factor, Fms-related tyrosine kinase 3 ligand), other cytokines (eg, tumor necrosis factor alpha [TNF-α]), and transcription factors (eg, interferon regulatory factor 8) are essential for DC development and differentiation.2 DCs themselves are major sources of cytokines, including interleukin-12 (IL-12) and TNF-α. These very same cytokines are aberrantly expressed in myelofibrosis (MF),3 which is also characterized by constitutive Janus kinase signal transducer and activator of transcription (JAK-STAT) activation.3 Therefore, DCs are either directly or indirectly involved in the inflammatory process that accompanies MF (see figure).
Ruxolitinib inhibition is not specific to mutant JAK2; it also targets wild-type JAK2 and JAK1 equipotently, as well as other kinases.4 The corresponding signaling pathways that are affected include JAK-STAT, mitogen-activated protein kinase extracellular-regulated kinase 1/2 (MAPK-ERK1/2), and phosphatidylinositol-3 kinase AKT (PI3K-AKT).5 These pathways are also used for transcriptional induction of DC-relevant cytokines. Therefore, it is not surprising to discover ruxolitinib-associated DC dysfunction; however, the particular observation highlights the risk for adverse off-target effects arising from nonspecific treatment strategies that target multiple cytokines and physiologically important pathways.
Disruption of physiologic JAK-STAT signaling, especially in the context of multi-JAK inhibition, also affects lymphocyte homeostasis.5 The composite DC-lymphocyte effect might partly explain the ruxolitinib-induced downregulation of inteleukin (IL)-6, IL-8, TNF-α, interferon-gamma, IL-1Rα, and other inflammatory cytokines in vivo (see figure).6 One could argue that such cytokine modulation might be of benefit for inflammation-associated diseases, including MF. However, such a treatment strategy ignores the basic principle of cancer therapy, which is selective suppression of the malignant clone. Instead, it encourages settling for symptom palliation as a treatment end point.
Ruxolitinib therapy in MF conjures limited benefit in terms of reversing bone marrow fibrosis or significantly reducing JAK2 V617F allele burden6 ; its value is mostly limited to relief of constitutional symptoms and reduction in spleen size. Even then, benefit comes at the cost of drug-associated anemia, thrombocytopenia, and cytokine-release syndrome during drug discontinuation.4,7 Other JAK inhibitors that are either more selective in targeting JAK28 or less likely to cause anemia9 are not necessarily spared from similar handicaps (ie, lack of selective anticlonal activity and association with nontrivial drug side effects, including gastrointestinal and neurologic complications).4 The observation by Heine et al1 further highlights the need to be vigilant about long-term ill effects.
Conflict-of-interest disclosure: The author declares no competing financial interests.