Key Points
Dietary ALA decreases platelet clearance in a mouse model of atherosclerosis.
ALA reduces platelet activation and tumor necrosis factor-alpha–converting enzyme-dependent GPIb cleavage.
Abstract
Previously we reported that dietary intake of alpha-linolenic acid (ALA) reduces atherogenesis and inhibits arterial thrombosis. Here, we analyze the substantial increase in platelet count induced by ALA and the mechanisms of reduced platelet clearance. Eight-week-old male apolipoprotein E knockout (ApoE−/−) mice were fed a 0.21g% cholesterol diet complemented by either a high- (7.3g%) or low-ALA (0.03g%) content. Platelet counts doubled after 16 weeks of ALA feeding, whereas the bleeding time remained similar. Plasma glycocalicin and glycocalicin index were reduced, while reticulated platelets, thrombopoietin, and bone marrow megakaryocyte colony-forming units remained unchanged. Platelet contents of liver and spleen were substantially reduced, without affecting macrophage function and number. Glycoprotein Ib (GPIb) shedding, exposure of P-selectin, and activated integrin αIIbβ3 upon activation with thrombin were reduced. Dietary ALA increased the platelet count by reducing platelet clearance in the reticulo-endothelial system. The latter appears to be mediated by reduced cleavage of GPIb by tumor necrosis factor-α–converting enzyme and reduced platelet activation/expression of procoagulant signaling. Ex vivo, there was less adhesion of human platelets to von Willebrand factor under high shear conditions after ALA treatment. Thus, ALA may be a promising tool in transfusion medicine and in high turnover/high activation platelet disorders.
Introduction
The platelet count is regulated by a complex interplay of thrombopoietin (TPO), pro- and antiapoptotic molecules Bak/Bcl, platelet activation, expression of procoagulant and proapoptotic factors including phosphatidylserine/phosphatidylethanolamine (PS/PE) and the caspase system, clustering and/or loss of specifically glycosylated receptors such as glycoprotein Ib (GPIb), and, finally, clearance by the reticuloendothelial system from the circulation.1-6
Long-chain omega-3 fatty acids (LCn3-FAs) exert beneficial cardiovascular effects on atherosclerosis and inflammation and have been demonstrated to induce antithrombotic and antiplatelet effects and to reduce mortality.7-13 Although the Alpha Omega Trial did not show a significantly different event rate among patients receiving LCn3-FA in addition to state-of-the-art therapy for myocardial infarction,14 previous studies and subgroup analyses demonstrated a beneficial effect of omega (ω)-3 FA.11,15 An inexpensive and abundantly available alternative to marine-derived ω-3 is the plant-derived alpha (α)-linolenic acid (ALA) that, by itself or by chain elongation to LCn3-FA EPA, DPA, and DHA, has a number of beneficial effects. However, these effects are somewhat less well characterized than those of the LCn3-FAs. Furthermore, some landmark studies investigating nutritional interventions (Mediterranean diet and nuts, walnuts in particular, as food additives) have been performed, where ALA is likely to contribute to the main beneficial effects.16-19
We recently reported on the mechanisms of ALA and its antiinflammatory, antiatherosclerotic, and antiplatelet effects.20,21 Dietary ALA was incorporated into various adipose tissues in a mouse model of atherosclerosis and switched the balance toward the antiinflammatory class of the thromboxane/prostacycline mediators.20 Interestingly, we have shown that the nutritional intake of ALA resulted in prolonged time to occlusion in a mouse model of carotid thrombosis.21
Given the reduction in platelet activation and based on the above-mentioned observations, we hypothesized that platelets could circulate longer in the blood stream due to reduced clearance signaling, thus increasing the net platelet counts. Preliminary observations of increased platelet counts in pilot experiments22 encouraged us to extend our studies to further analyze this hypothesis and its potential mechanisms, with eventual translational applications of this finding.
Methods
Animals and diet
Eight-week-old male C57/BL6 apolipoprotein E knockout (ApoE−/−) mice were fed a 0.21g% cholesterol diet containing either high ALA (7.3g%, D06080702; Research Diets, New Brunswick, NJ; n = 20) or low ALA (0.03g%, D06080701; Research Diets; n = 20) for 16 weeks. ALA was given as flaxseed oil; cocoa butter was used as the substitute in the control group.20 At age 24 weeks, animals were killed and tissues harvested. All animal experiments were approved by the local ethics committee for animal protection.
Blood analyses
Mice were fasted over night before blood was drawn. Plasma and red cells were stored at −80°C. Total blood cell count was performed on a ScilVet ABCplus (Horiba, Kyoto, Japan) using EDTA-anticoagulated blood.
Bleeding times
Bleeding times were measured according to Jansen et al.23 Mice were anesthetized by intraperitoneal injection of sodium pentobarbital; the last 5 mm of the tail was cut with a razor. Tails were placed in saline at 37°C, and the time to bleeding cessation was determined with a stopwatch.
Reticulated platelets
Newly synthesized platelets (reticulated platelets) were determined using thiazole orange staining. Briefly, 5 μL of whole blood were mixed with 50 μL thiazole orange solution (Sigma-Aldrich, St. Louis, MO; 0.1 μg/mL in methanol) and incubated for 15 minutes in the dark. After staining, platelets were fixed by addition of 1 mL 1% paraformaldehyde in phosphate-buffered saline (PBS) and placed on ice. Samples were immediately analyzed on a FacsCanto flow cytometer (BD Bioscience, Heidelberg, Germany), and the percentage of positive platelets calculated with FacsDiva software.
Plasma glycocalicin and glycocalicin index
Plasma glycocalicin was assayed using enzyme-linked immunosorbent assay. Briefly, 96-well plates (Costar) were coated with the antibody 7A9 (directed against the extracellular part of GPIb, a kind gift of Bernard Nieswandt, Wurzburg, Germany) at 5 μg/mL in 50 mM NaHCO3 and incubated at 4°C overnight. The next day plates were washed 3 times with 0.1% PBS/Tween 20 (PBST) and then blocked with 5% bovine serum albumin (BSA)–5% milk in PBS for 2 hours at 37°C. After washing again 3 times with PBST, plates were incubated with standards; samples were diluted in 1% BSA–1% milk in PBS and incubated for 1 hour at 37°C. Plates were washed again 3 times and incubated with the horseradish peroxidase–conjugated detection antibody 15E3 for 30 minutes at 37°C. Plates were then washed 4 times with PBST and incubated with 3,3′,5,5′-tetramethylbenzidine substrate solution (BD Bioscience) for 5 minutes. The reaction was stopped with 0.5 M sulfuric acid, and absorbances were read at 450 nm with a Spectramax plate reader (Molecular Probes). To determine the glycocalicin index, absolute plasma concentrations were multiplied for the platelet count that was normalized to 250 000/μL.24
Plasma TPO, interleukin-6, and thromboxane B2
Plasma concentrations of TPO, interleukin-6, and thromboxane B2 (TXB2) were determined on samples stored at −80°C by multiplex assay (Cytolab, Dallikon, Switzerland).
Platelet activation
For platelet activation studies, washed platelets were obtained from citrate-anticoagulated blood after mice euthanasia as described elsewhere25 and resuspended in platelet buffer A (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 140 mM NaCl, 3 mM KCl, 0.5 mM MgCl2 hexahydrate, 0.5 mM NaHCO3, 10 mM glucose; pH = 7.4). Platelets were activated with thrombin (0.05 and 0.1 U/mL) or collagen (final concentration 5 μg/mL) in flow cytometry tubes and simultaneously incubated with specific anti–P-selectin and antiactivated integrin αIIbβ3 antibodies (Emfret Analytics, Eibelstadt, Germany) for 15 minutes in the dark. Reaction was stopped by addition of 200 μL PBS. Samples were analyzed immediately on a FacsCanto flow cytometer (BD Bioscience).
For detection of PS, washed platelets were incubated with thrombin and annexin V–fluorescein isothiocyanate (FITC; Roche, Basel, Switzerland) for 15 minutes. The reaction was stopped by the addition of PBS, and samples were analyzed by flow cytometry.
Platelet survival
Platelet survival was determined by in vivo platelet labeling. Briefly, mice were injected in the tail vein with ×488 (an immunoglobulin G derivative directed against the β subunit of the complex GPIb; Emfret Analytics), 0.15 μg/g body weight. A small blood sample (5 μL) was taken at various time points (2, 24, 48, 72, and 96 hours) and mixed with buffer A containing Aster Jandl citric-based anticoagulant (85 mM sodium citrate dihydrate, 69 mM citric acid, 20 mg/mL glucose; pH 4.6). Diluted platelet-rich plasma was isolated after centrifugation for 8 minutes at 125 g and analyzed immediately on a FacsCanto flow cytometer (BD Bioscience). Positive platelets at 2 hours were set as 100% and all subsequent time points were calculated accordingly.
Tissue processing of liver and spleen
Spleens and livers from 3 mice/group were stored in optimal cutting temperature medium at −80°C. Frozen sections (5 μm) were obtained and fixed in ice cold acetone for 30 minutes. Slides were blocked with 10% goat serum in PBS for 1 hour at room temperature, then incubated with the rat anti-mouse α2b (CD41) antibody (Ab) and the rabbit anti-mouse CD68 Ab (both Abcam, Cambridge, UK), diluted in 1% BSA in PBS overnight at 4°C. After washing in PBS, sections were incubated with a goat anti-rat Ab conjugated with Alexa 647 and a goat anti-rabbit Ab conjugated with Alexa 488 for 1 hour at room temperature. Slides were washed with PBS, and coverslips were mounted with DAPI-mounting medium (Dako, Glostrup, Denmark). Slides were stored at −20°C until analysis. Single images were taken using an Olympus BX51 microscope equipped with an Olympus DP70 camera (Olympus, Tokyo, Japan) and overlayed with Image J software (NIH).
GPIbα shedding by tumor necrosis factor-alpha– converting enzyme
Cleavage of the GPIbα extracellular domain after platelet activation by thrombin was monitored by flow cytometry. Washed platelets from mice were activated with thrombin (0.5 or 1 U/mL). Samples were stained for the platelet marker α2b (CD41) and for GPIb with fluorophore-conjugated antibodies (Emfret Analytics) and then analyzed on a FacsCanto flow cytometer (BD Bioscience). GPIb cleavage was expressed as % fluorescence vs the resting state on CD41-positive cells. In the experiments using inhibitors, platelets were incubated simultaneously with thrombin and either the broad-spectrum metalloprotease inhibitor GM-6001 or the specific inhibitor for tumor necrosis factor-alpha–converting enzyme (TACE) (TAPI-1), p38 (SB203580), PI3K (wortmannin and LY294002), or PKC (calphostin C) as well as the FITC-conjugated GPIb antibody, then analyzed as described above.
Megakaryocyte colony-forming unit assay
Femura from killed mice were harvested and cells from bone marrow flushed with 3 mL Iscove’s modified Dulbecco’s medium (Lonza, Basel, Switzerland) with 2% FBS with a syringe attached to a 21-G needle. Cells were counted and resuspended at a concentration of 2.2 × 106 cells/mL. Next, 100 μL of the cell suspension was added to 2 mL MegaCult medium containing cytokine (Stemcell, Vancouver, BC, Canada), then 1.2 mL of cold collagen were added and cells vortexed and plated in duplicate into a chamber slide. Cells were grown for 7 days, then the chamber slides were fixed in ice-cold acetone and megakaryocyte colony-forming unit (MK-CFU) stained for acetylcholinesterase activity following the MegaCult protocol. Slides were scored under a light microscope (Leica, Wetzlar, Germany).
Platelet adhesion to vWF
Platelet adhesion to von Willebrand Factor (vWF) was assessed with the Bioflux system (Fluxion, San Francisco, CA). Blood from healthy donors was incubated with ALA (Cayman Chemicals, Denver, CO) at concentrations of 7.5, 15, and 30 μM or with vehicle (ethanol) for 1 hour at room temperature and platelets stained with calcein (Enzo Life Science, Lausen, Switzerland). Bioflux plates were coated with human vWF at 100 μg/mL (Haematological Technologies, Essex Junction, VT), then blocked with 5% BSA in PBS for 10 minutes. Plates were placed on an inverted microscope and 1 mL blood applied to the inlet well. A shear force of 200 dynes/cm2 was applied, and the platelets allowed to adhere to vWF for 10 minutes. Images were taken at the end of the experiment, and the platelet-covered area was measured using the Bioflux software.
Statistics
Values are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using an unpaired 2-tailed Student t test or a 1-way analysis of variance with Bonferroni post-hoc test. Significance was accepted at P < .05. Values are expressed as mean ± SEM.
Results
Dietary ALA increases platelet counts without affecting bleeding times
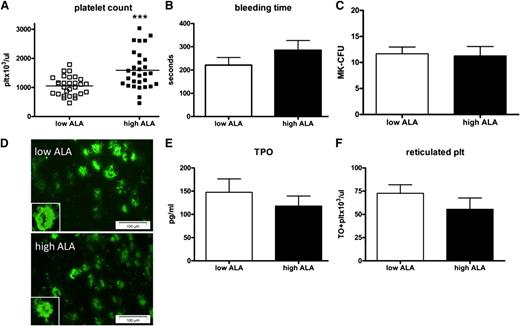
To assess the effects of dietary ALA on platelet number and function, ApoE−/− mice were given a high-fat diet supplemented with low or high ALA. After 16 weeks on the diet, the platelet count in the mice fed high ALA had increased to 1591 ± 119 plt/μL compared with 1052 ± 60 plt/μL for the low-ALA mice (Figure 1A). Despite increased platelet count, bleeding times did not differ significantly (Figure 1B).
Dietary ALA increases the platelet count but not their production. Platelet count in ApoE−/− mice was significantly increased after 16 weeks on a high-ALA diet (A; n = 30; P = .0002). In contrast, tail bleeding time was prolonged but not significantly (B; n = 5, P > .05). Megakaryocytes from bone marrow were harvested and assayed for CFUs, revealing no difference between low- and high-ALA mice (C; n = 3; P > .05). Bone marrow sections were stained for the megakaryocyte marker CD41; 3 images per section were taken at random and CD41+ cells scored (D; n = 3, P > .05; magnification 20×). Plasma TPO levels were not significantly different in the high-ALA group (E; n = 13; P > .05). Reticulated platelets representing newly synthesized platelets were not different between the groups (F; n = 15; P > .05).
Dietary ALA increases the platelet count but not their production. Platelet count in ApoE−/− mice was significantly increased after 16 weeks on a high-ALA diet (A; n = 30; P = .0002). In contrast, tail bleeding time was prolonged but not significantly (B; n = 5, P > .05). Megakaryocytes from bone marrow were harvested and assayed for CFUs, revealing no difference between low- and high-ALA mice (C; n = 3; P > .05). Bone marrow sections were stained for the megakaryocyte marker CD41; 3 images per section were taken at random and CD41+ cells scored (D; n = 3, P > .05; magnification 20×). Plasma TPO levels were not significantly different in the high-ALA group (E; n = 13; P > .05). Reticulated platelets representing newly synthesized platelets were not different between the groups (F; n = 15; P > .05).
TPO and bone marrow macrophages are unchanged after a high-ALA diet
To further analyze the mechanisms that relate to the difference in platelet count in the high ALA mice, bone marrow megakaryocytes were evaluated. In a CFU assay, megakaryocytes isolated from femura showed no difference in the number of CFUs between low- and high-ALA mice (Figure 1C). Staining of bone marrow sections for the megakaryocyte marker CD41 also showed no difference in the number of these cells in the 2 groups (Figure 1D). When plasma levels of TPO were assayed, no difference was found between the 2 groups of mice (Figure 1E). Both sets of results ruled out the possibility that ALA could increase platelet production by increasing megakaryocyte number in the bone marrow or by inducing TPO production. Accordingly, reticulated platelets, an independent measure of platelet production, were numerically lower in the high-ALA group, but the difference was not significant (Figure 1F). Interestingly, there was a decrease of plasma TXB2 and PGE2 in the high-ALA animals, but it did not reach statistical significance due to an important interindividual variability in the samples (data not shown).
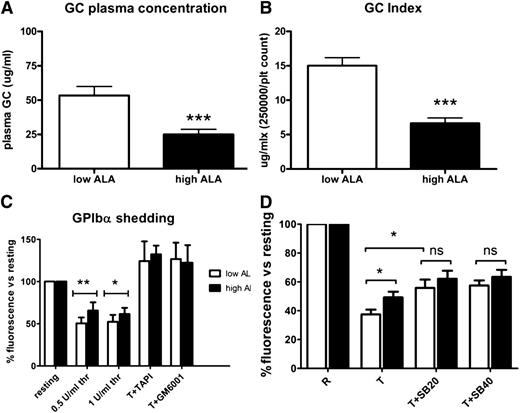
ALA reduces platelet turnover as demonstrated by glycocalicin and glycocalicin indices
Plasma glycocalicin was used to determine platelet turnover, as it has been shown to correlate with platelet production/clearance.24,26 Plasma glycocalicin was significantly lower in the mice fed the high-ALA diet (25 ± 4 μg/mL compared with 53 ± 27 μg/mL; Figure 2A). The glycocalicin index (plasma glycocalicin normalized to the platelet count) was also significantly lower in the high-ALA group (6.6 ± 3 compared with 15 ± 5; Figure 2B).
ALA reduces platelet turnover and TACE-mediated GPIb shedding. Plasma glycocalicin (a marker for platelet turnover) as well as the glycocalicin index (A-B; n = 17; P < .001) were significantly decreased in mice fed the high-ALA diet. GPIb cleavage after platelet activation with 0.5 and 1 U/mL thrombin was monitored by flow cytometry of washed platelets. Results are expressed as percentage of fluorescence vs resting state (C; n = 10; *P = .027; **P = .003). In the presence of the specific TACE inhibitor TAPI-1 or the broad metalloprotease inhibitor GM6001, cleavage was completely abrogated (C; n = 5). Addition of the p38 specific inhibitor SB203580 (at concentrations of 20 and 40 μM) blunted the difference between low- and high-ALA platelets, indicating that the TACE inhibition by ALA is p38 dependent (D; n = 8; *P < .05).
ALA reduces platelet turnover and TACE-mediated GPIb shedding. Plasma glycocalicin (a marker for platelet turnover) as well as the glycocalicin index (A-B; n = 17; P < .001) were significantly decreased in mice fed the high-ALA diet. GPIb cleavage after platelet activation with 0.5 and 1 U/mL thrombin was monitored by flow cytometry of washed platelets. Results are expressed as percentage of fluorescence vs resting state (C; n = 10; *P = .027; **P = .003). In the presence of the specific TACE inhibitor TAPI-1 or the broad metalloprotease inhibitor GM6001, cleavage was completely abrogated (C; n = 5). Addition of the p38 specific inhibitor SB203580 (at concentrations of 20 and 40 μM) blunted the difference between low- and high-ALA platelets, indicating that the TACE inhibition by ALA is p38 dependent (D; n = 8; *P < .05).
ALA reduces TACE-mediated cleavage of glycocalicin and preserves GPIb expression
To further elucidate potential mechanisms, we analyzed the TACE-mediated cleavage of the extracellular portion of GPIbα upon platelet activation. This is an important negative feedback mechanism by which platelets are rendered progressively less capable of adhering to vWF and of aggregating, thus avoiding excessive platelet activation.27,28 Since we found a reduction of plasma glycocalicin following a high-ALA diet (Figure 2A), we hypothesized that GPIbα cleavage by TACE following platelet stimulation would be reduced. The results were in line with our previous finding: although GPIbα levels on resting platelets were not different in the 2 groups (data not shown), GPIbα shedding was significantly reduced after platelet activation with both thrombin concentrations used (Figure 2C). When the specific TACE inhibitor TAPI-1 was added, GPIb cleavage was completely abrogated, showing that receptor cleavage upon thrombin stimulation is TACE dependent (Figure 2C). The use of a broad-spectrum metalloprotease inhibitor (GM-6001) abrogated GPIb shedding as well (Figure 2C). To further elucidate possible mechanisms of TACE inhibition by ALA, the same experiment was performed in the presence of specific inhibitors of p38, PI3K, and PKC (supplemental Figure 1B). Only the p38 inhibitor SB203580 was able to reduce TACE-dependent GPIb cleavage, blunting the difference between the low- and high-ALA group (Figure 2D). This demonstrates a role for p38 in mediating TACE inhibition by ALA.
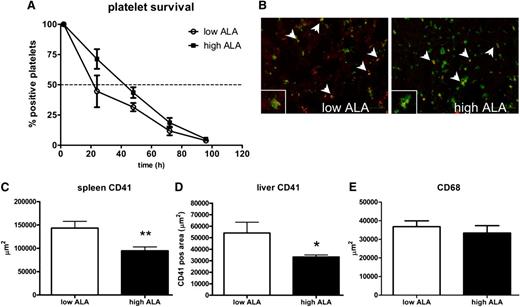
Platelet half-life is increased whereas platelet clearance in the liver and spleen are reduced by ALA
Platelet survival was determined by in vivo platelet labeling, which allows their clearance to be analyzed over 5 days. Platelet lifespan was not increased upon ALA treatment, but platelet half-life was prolonged in the high-ALA group, even though it did not reach statistical significance (Figure 3A). Since it has been shown that platelet lifespan is regulated by the pro- and antiapoptotic molecules Bax and BclXL, we also analyzed the expression of these molecules by western blotting. However, we did not find a difference in the platelets from the 2 groups of mice, which is in agreement with our result (data not shown). Analysis of activation of caspase-3, a mediator of apoptosis, also showed no difference between the 2 groups (supplemental Figure 1A).
Platelet clearance in spleen and liver is reduced after a high-ALA diet. Platelet half-life was increased in the high-ALA compared with the low-ALA group (A; n = 6; P = .07). Liver cryosections were stained for the platelet marker CD41 (red) and the macrophage marker CD68 (green); images were taken with an Olympus microscope (B; magnification 20×; scale bar 100 μm). Colocalization is shown by a merge of the 2 fluorescent signals (yellow, arrows). Spleen and liver cryosections were also analyzed for the platelet marker CD41 (green); the positive area quantified with the software AnalySIS 3.1 (Soft Image Solutions) and was found to be significantly reduced in the high-ALA mice in both organs (C-D; n = 3; *P = .03; **P = .005). Macrophage number in the spleen and liver was not affected by the ALA treatment as shown by quantification of the CD68-positive area (E; n = 3; P > .05).
Platelet clearance in spleen and liver is reduced after a high-ALA diet. Platelet half-life was increased in the high-ALA compared with the low-ALA group (A; n = 6; P = .07). Liver cryosections were stained for the platelet marker CD41 (red) and the macrophage marker CD68 (green); images were taken with an Olympus microscope (B; magnification 20×; scale bar 100 μm). Colocalization is shown by a merge of the 2 fluorescent signals (yellow, arrows). Spleen and liver cryosections were also analyzed for the platelet marker CD41 (green); the positive area quantified with the software AnalySIS 3.1 (Soft Image Solutions) and was found to be significantly reduced in the high-ALA mice in both organs (C-D; n = 3; *P = .03; **P = .005). Macrophage number in the spleen and liver was not affected by the ALA treatment as shown by quantification of the CD68-positive area (E; n = 3; P > .05).
Senescent platelets are removed from the circulation by the reticuloendothelial system of the spleen and liver by recognition of specific residues in glycosylated proteins.3,4 In order to analyze platelet clearance in these 2 organs, cryosections were stained for the platelet marker α2b. In both cases, the CD41-positive area was decreased in the high-ALA group: in the spleen, 9472 ± 838 μm2 compared with 14 348 ± 1442 μm2; in the liver, 3322 ± 180 μm2 compared with 5409 ± 934 μm2 (Figure 3C-D). Liver sections were also costained for the macrophage marker CD68, and the overlay of the 2 fluorescences revealed colocalization of the signal, showing platelet removal by macrophages (Figure 3B arrows). In order to show that this phenomenon was due to an inhibition of macrophage function, the CD68-positive area was quantified and found not to be different in the 2 groups (Figure 3E). Macrophage function was also assessed by injection of fluorescently labeled platelets isolated from untreated animals into ALA-treated mice, followed by harvesting of livers and spleens after 2 hours and analysis of cryosections (supplemental Figure 2). This experiment also showed no difference in platelet uptake, demonstrating that macrophage function was not altered by ALA treatment and was not responsible for the reduced platelet clearance observed.
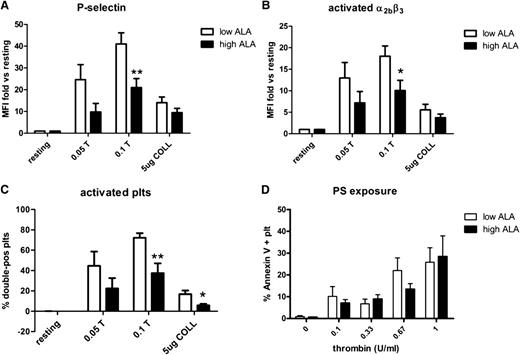
ALA reduces exposure of P-selectin and activated integrin αIIbβ3
We analyzed exposure of PS/PE, P-selectin, and active αIIbβ3 integrin on washed platelets from the 2 groups of mice after stimulation with thrombin (0.05 or 0.1 U/mL) or collagen (5 μg/mL). P-selectin exposure and integrin activation were both significantly reduced after stimulation with 0.1 U/mL thrombin. However, after collagen stimulation, the reduction did not reach statistical significance (Figure 4A-B). Analysis of the platelets that were positive for both P-selectin and activated integrin also showed a significant reduction in the high-ALA group after stimulation with either thrombin or collagen (Figure 4C). This indicates an effective inhibition of the intracellular pathways leading to granule release and calcium mobilization. Binding of annexin V was also reduced, but the difference did not reach statistical significance (Figure 4D).
Expression of P-selectin and integrin α2bβ3 activation is reduced after a high-ALA diet. Washed mouse platelets were activated with thrombin (0.05 and 0.1 U/mL) or collagen (5 μg/L) and stained with specific fluorophore-conjugated antibodies for P-selectin (A) and the activated form of the integrin α2bβ3 (B). Samples were analyzed by flow cytometry. In both cases there was a reduced positivity in platelets from high-ALA mice (n = 8; *P = .045; **P = .01). The percentage of double-positive platelets was also found to be significantly lower after a high-ALA diet (C; n = 8; *P = .03; **P = .004). Binding of annexin V to PS/PE was decreased overall in high-ALA mice but did not reach statistical significance (D; n = 5; P > .05).
Expression of P-selectin and integrin α2bβ3 activation is reduced after a high-ALA diet. Washed mouse platelets were activated with thrombin (0.05 and 0.1 U/mL) or collagen (5 μg/L) and stained with specific fluorophore-conjugated antibodies for P-selectin (A) and the activated form of the integrin α2bβ3 (B). Samples were analyzed by flow cytometry. In both cases there was a reduced positivity in platelets from high-ALA mice (n = 8; *P = .045; **P = .01). The percentage of double-positive platelets was also found to be significantly lower after a high-ALA diet (C; n = 8; *P = .03; **P = .004). Binding of annexin V to PS/PE was decreased overall in high-ALA mice but did not reach statistical significance (D; n = 5; P > .05).
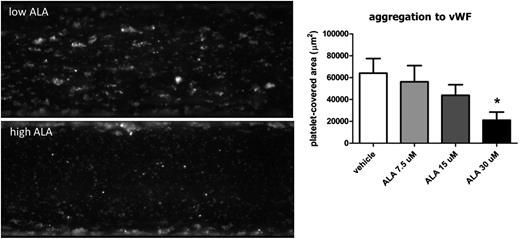
ALA dose-dependently reduces platelet adhesion and aggregation to vWF under high shear flow
To examine the effect of ALA on vWF–GPIb binding, we performed experiments of platelet adhesion to vWF under high shear flow. We used human blood from healthy donors in order to translate our results from the animal model into a clinically significant scenario. Incubation of whole blood with ALA in increasing concentrations demonstrated a dose-dependent inhibition of platelet adhesion to vWF and platelet aggregation after 10 minutes of flow compared with vehicle (Figure 5; supplemental Videos 1 and 2).
ALA reduces platelet adhesion and aggregation to vWF under flow. Blood from human healthy donors was incubated with 7.5, 15, and 30 μM ALA or vehicle (ethanol) for 1 hour. Adhesion to vWF was quantified at a shear rate of 2500 s−1 for 10 minutes with the Bioflux software. There was a dose-dependent reduction of platelet adhesion and aggregation that was significant for the highest ALA concentration used (D; n = 6; *P = .02).
ALA reduces platelet adhesion and aggregation to vWF under flow. Blood from human healthy donors was incubated with 7.5, 15, and 30 μM ALA or vehicle (ethanol) for 1 hour. Adhesion to vWF was quantified at a shear rate of 2500 s−1 for 10 minutes with the Bioflux software. There was a dose-dependent reduction of platelet adhesion and aggregation that was significant for the highest ALA concentration used (D; n = 6; *P = .02).
Discussion
In the last decade, a number of clinical trials have been performed on the efficacy of ω-3 fatty acids as cardioprotective agents, with discordant results.11,29,30 A recent metaanalysis found ALA to be associated with a lower risk of cardiovascular disease.31,32
Here we show that dietary ALA consistently increases the platelet count to a biologically relevant degree (ie, 51%) in the same mouse model as atherosclerosis (ApoE−/− mice) in which we previously documented a reduction in atherosclerosis by this dietary intervention.20 The increased number of platelets did not translate into a shortened bleeding time; on the contrary, bleeding time tended to be prolonged in mice fed a high-ALA diet. Thus, the increased platelet count seems to compensate for the reduced aggregation response under these dietary conditions. We further elucidated the mechanisms involved. The reduction in plasma glycocalicin and glycocalicin index as well as the slightly reduced number of reticulated platelets support the hypothesis of unchanged, stable platelet production and reduced platelet clearance by the reticuloendothelial system. Immunofluorescence staining of liver and spleen sections from animals on a high-ALA diet confirms a striking reduction of CD41 fluorescence, consistent with our hypothesis of a reduced clearance induced by dietary ALA. In agreement with this observation, plasma TPO concentrations in the 2 groups were comparable, and the number of MK-CFUs in the bone marrow were identical. In addition, ALA did not alter the homoeostasis of the apoptotic pathway nor did it increase caspase-3 activity. Since macrophages could also be affected by ALA, thus leading to a reduced platelet uptake, we tested liver macrophage numbers in ALA-treated animals and found them to be the same in the 2 groups. Injection of fluorescently labeled platelets from untreated mice into ALA-treated mice also showed no difference in platelet uptake in liver and spleen, confirming that macrophage function was not altered. This result is in agreement with our previous work that showed that macrophage recruitment into atherosclerotic plaque was not affected by ALA20 and confirms our hypothesis that the reduced platelet activation is the basis for the reduced clearance. The reduced expression of the active conformation of integrin α2bβ3 (platelet CD41) and of P-selectin (CD62-P) upon activation with thrombin or collagen may also lead to reduced clearance from the circulation.
We also provide evidence that platelet activation by thrombin or collagen resulted in a reduced cleavage of GPIb by the enzyme TACE, thus reducing the phagocytosis signal in the reticulo-endothelial system of the liver and spleen. To our knowledge, this is the first time that an ω-3 FA is reported to inhibit TACE activity. Since TACE has been reported to be directly activated by p38,33 and previous work from our group has demonstrated an inhibition of p38 phosphorylation by ALA in platelets,21 we hypothesized that ALA could inhibit TACE by reducing p38 activation in response to thrombin stimulation. By using a specific p38 inhibitor, we were able to reduce thrombin-induced GPIb cleavage and blunt the difference observed between the low- and high-ALA mice. Our results collectively demonstrate that thrombin-induced GPIb shedding is specifically mediated by TACE and that ALA inhibits TACE activity via p38, although the involvement of other pathways cannot be excluded.
To translate our results into a functional assay and into a potentially clinically relevant setting, we analyzed platelet adhesion and aggregation under flow onto vWF in human blood preincubated with ALA. After an incubation as short as 1 hour, ALA reduced platelet adhesion and aggregation on von Willebrand factor, suggesting a reduced platelet activatability under a number of conditions (thrombin or collagen-activation, high shear flow).
Taken together, our data indicate an increased platelet count due to reduced clearance but also reduced activatability of the platelets; however, no evidence of an effect on the apoptosis pathway could be observed. The finding is novel and, to our knowledge, this is the first time this observation has been made. The timing of the platelet count elevation appears of interest; in a preliminary series of experiments, we found that no increase can be observed until 6 weeks after the nutritional intervention. We purposely chose the ApoE−/− model because it mimics the clinical situation of atherosclerosis with increased platelet activation. Whether the ApoE−/− model represents a low-grade inflammatory model combined with a Western-type diet that is improved by ALA is not clearly understood. Preliminary data indicate that wild-type mice seem to increase their platelet counts more modestly. However, a number of data indicate a consistent reaction in the human model, among them the data on human platelet adhesion under flow.
In conclusion, we show that dietary ALA reduces platelet clearance by reducing platelet activation and turnover and that ALA inhibits TACE-mediated GPIb cleavage via inhibition of p38. These findings could be of interest in transfusion medicine where they may be applicable in platelet preservation and in diseases with conditions of high platelet turnover and/or pathological platelet activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to P. Blyszczuk for the valuable comments and advice.
This work was supported by the Swiss National Science Foundation (grant 320080-1042.02; J.H.B.), the University Research Priority Program, Integrative Human Physiology, at the University of Zurich (T.F.L.), the Swiss Heart Foundation (J.H.B.), and the CARDIO-Foundation (J.H.B.).
Authorship
Contribution: S.S. performed the experiments and wrote the manuscript; M.F.R. performed the experiments; C.L. performed the experiments; T.F.L. and C.M.M. revised the manuscript; and J.H.B. designed the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.L. is Center for Regenerative Medicine, University Hospital Zurich, Switzerland.
Correspondence: Juerg Hans Beer, Laboratory for Platelet Research, University of Zurich, Switzerland, and Department of Internal Medicine, Cantonal Hospital of Baden, Im Ergel 1, 5404 Baden, Switzerland; e-mail: hansjuerg.beer@ksb.ch.