Key Points
Treatment with lenalidomide induces long-lasting responses.
Lenalidomide can produce a sustained increase in immunoglobulin levels and a sustained normalization in circulating T cells.
Abstract
We evaluated long-term outcomes of 60 patients with chronic lymphocytic leukemia treated with an initial therapy of lenalidomide. At a median follow-up of 4 years, time-to-treatment failure has not been reached and overall survival is 82%. Thirty-five (58%) patients had a response lasting >36 months (long-term responders [LTRs]). Best LTR responses consisted of 25 (71%) complete remissions and 10 (29%) partial remissions. In addition to clinical responses, an increase in IgA, IgG, and IgM levels of >50% from baseline was reported in 61%, 45%, and 42% of LTRs. Normalization in the percentage of CD4+ and CD8+ cells and T-cell numbers was observed in 48%, 71% and 99% of LTRs. Compared with other patients in the study, LTRs had lower baseline plasma levels of β-2-microglobulin, were more likely to have trisomy 12, and less likely to have deletion 17p. This trial was registered at http://clinicaltrials.gov as # NCT00535873.
Introduction
The best initial treatment of elderly patients with chronic lymphocytic leukemia (CLL) has not yet been defined.1 We investigated the activity of lenalidomide,2-5 an oral immunomodulatory agent,6-9 as frontline treatment of older patients with CLL, and observed an overall response rate of 56% with 2-year overall and progression-free survival durations of 88% and 60%.10 Because response duration and long-term survival are important clinical endpoints, we sought to report the long-term outcome of this trial.
Study design
Sixty treatment-naive patients with symptomatic CLL11 were enrolled in a phase 2 study of lenalidomide at The University of Texas MD Anderson Cancer Center. Patients were included in a study approved by the Institutional Review Board of the MD Anderson Cancer Center and conducted in accordance with the Declaration of Helsinki. Treatment consisted of lenalidomide (5 mg daily by mouth) continuously. After 2 28-day cycles, the dose of lenalidomide could be escalated (by increments of 5 mg per cycle to 25 mg daily).10,12 The patients remained on treatment until disease progression. Clinical and laboratory assessment were performed every 6 months.11 Intracellular cytokine synthesis by activated T cells was measured as previously described.13,14 Measured cytokines were: interleukin (IL)-2, IL-6, IL-8, IL-10, IL-12p70, interferon-γ, tumor necrosis factor-α, soluble IL-6 receptor, soluble tumor necrosis factor receptor, soluble vascular endothelial growth factor receptor-1, -2 and -3, fibroblast growth factor-β, chemokine (C-C motif) ligand 3, and IL-1Rα. As median time-to-treatment failure was 30 months, patients who had a response lasting more than 36 months were defined as long-term responders (LTRs). Their clinical and laboratory features were compared with those of the rest of the study population using nonparametric and χ-square tests. Differences were considered to be significant if P ≤ .05.
Results and discussion
At a median follow-up of 4 years, median time-to-treatment failure has not been reached, and overall survival is 82%. Thirty-five (58%) of the 60 patients were LTRs. Best responses among the LTRs consisted of 25 patients with complete remission (CR: 71%), including 5 patients with no evidence of residual disease at bone marrow evaluation (minimal residual disease [MRD]-negative CR), and 10 patients with partial remission (PR: 29%). At the time of the initial report,10 the median time to achieve a PR or better was 18 months. At the time of this analysis, median time to best response was 25 (3-52) months. As patients continued on therapy with lenalidomide, we compared the quality of responses at 18 months and after 36 months. Twelve patients with a PR improved their response to a CR, 8 patients with stable disease improved their response to PR, and 2 additional patients achieved MRD-negative CR. Median time-to-treatment failure has not been reached for the LTRs, after a median follow-up of 48 (37-60) months. All (100%) of the LTRs are alive. Twenty-five of the LTRs are still on therapy. The median daily dose of lenalidomide was 5 mg (range: 2.5-10 mg) for the LTRs. Fourteen (40%) LTRs needed a dose reduction within 18 months of therapy and 19 (54%) LTRs after 18 months. Hematologic toxicity was the reason for dose reduction within and after 18 months in 93% and 53% of LTRs, respectively. Ten of the LTRs have discontinued lenalidomide; reasons for treatment discontinuation were: progression in 1 patient (after 43 months), toxicity in 6 patients (deep venous thrombosis after 41 months in 1 patient; moderate neuropathy after 30 and 39 months in 2 patients, respectively; persistent fatigue after 23 months in 1 patient; moderate weight loss after 5 months in 1 patient; and immune thrombocytopenia after 11 months in 1 patient), infectious complications in 1 patient (sepsis after 12 months), second malignancy (invasive squamous cell carcinoma of the skin after 26 months) in 1 patient, and change of institution in 1 patient. Two of these patients have progressed.
In patients with CLL, treatment with lenalidomide is often associated with myelosuppression during the initial months of treatment, but long-term hematologic toxicity has not been reported owing to the limited follow-up time in the published studies. Therefore, we reviewed the trend in absolute neutrophil count, hemoglobin level, and platelet count in the LTRs. The 35 LTRs experienced grade (G) >1 neutropenia (G3-4 in 12 patients [34%]) during the first 12 months of therapy. The neutropenia was later resolved in 29 (83%) of the patients, mostly through dose reduction. All G3-4 neutropenia resolved. Short-term growth factor support was required by 17% of patients. A recovery in hemoglobin levels and platelets count to normal range was reported in 100% and 77% of LTRs, respectively.
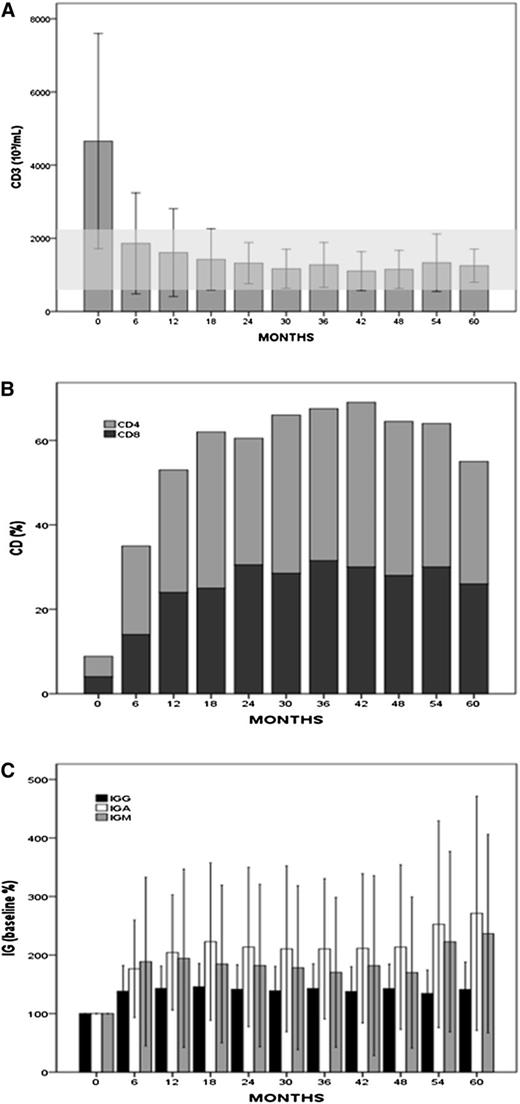
As reported at the time of the initial analysis of this trial, treatment with lenalidomide induced an increase in plasma levels of IgA, IgG, and IgM (Figure 1). A significant increase (P < .001) in IgA, IgG, and IgM of greater than 50% from baseline was observed, and sustained for longer than 36 months in 61%, 45%, and 42% of LTRs, respectively. Baseline IgG, IgA, and IgM were less than the normal range in 15 (43%), 14 (40%), and 22 (63%) LTRs and they normalized in 58%, 57%, and 45% of them, respectively. Treatment with lenalidomide was also associated with sustained (36 months or longer) normalization of the absolute number of circulating T cells (CD3+) and the percentage of CD4+ and CD8+ cells in 99%, 48%, and 71% of LTRs, respectively.
Changes in T-cell numbers and plasma immunoglobulin levels during therapy with lenalidomide. (A) Peripheral CD3+ T-cell numbers (mean values ± 1 standard deviation) measured at baseline and during treatment with lenalidomide in 31 LTRs. (B) Percentages of CD4 and CD8+ measured at baseline and during treatment with lenalidomide in 31 LTRs. Baseline median absolute value of CD4 and CD8 was 271 and 176 × 103/mL, respectively, and after 60 months increased to 456 and 362 × 103/mL, respectively. (C) Increase in plasma immunoglobulin measured in 31 LTRs during treatment with lenalidomide when compared with baseline (P < .001) . Values are expressed as percentage change from baseline, mean values ± 1 standard deviation. Median baseline were IgG, 729 (180-3560) mg/dL; IgA, 91 (15-223) mg/dL; and IgM, 27 (0-485) mg/dL. Baseline IgG, IgA, and IgM levels were less than the normal range in 15 (43%), 14 (40%), and 22 (63%) LTRs, and later normalized in 58%, 57%, and 45% of them, respectively.
Changes in T-cell numbers and plasma immunoglobulin levels during therapy with lenalidomide. (A) Peripheral CD3+ T-cell numbers (mean values ± 1 standard deviation) measured at baseline and during treatment with lenalidomide in 31 LTRs. (B) Percentages of CD4 and CD8+ measured at baseline and during treatment with lenalidomide in 31 LTRs. Baseline median absolute value of CD4 and CD8 was 271 and 176 × 103/mL, respectively, and after 60 months increased to 456 and 362 × 103/mL, respectively. (C) Increase in plasma immunoglobulin measured in 31 LTRs during treatment with lenalidomide when compared with baseline (P < .001) . Values are expressed as percentage change from baseline, mean values ± 1 standard deviation. Median baseline were IgG, 729 (180-3560) mg/dL; IgA, 91 (15-223) mg/dL; and IgM, 27 (0-485) mg/dL. Baseline IgG, IgA, and IgM levels were less than the normal range in 15 (43%), 14 (40%), and 22 (63%) LTRs, and later normalized in 58%, 57%, and 45% of them, respectively.
As treatment in this trial was indefinitely continued, we reviewed lenalidomide-related late toxicities in LTRs. Seven patients reported such toxicities, consisting of grade G1-2 diarrhea in 2 patients, G1 peripheral neuropathy in 3 patients, and G3-4 infections in 2 patients (bacterial pneumonia); these toxicities did not require treatment discontinuation. Among the LTRs, 1 patient developed several skin lesions shown to be in situ squamous and basal cell carcinomas, and these were treated by local excision.
We compared the clinical outcomes of the LTRs with those of the 25 patients that had no response or a response lasting <36 months (defined as short-term responders [STRs]). Responses among the STRs consisted of 4 CRs (2 MRD-negative) and 3 PRs. Eighteen patients failed to achieve a response. Median time-to-treatment failure in the STRs was 6 (range: 5-7) months. Twenty-one of the STRs received another treatment after a median time of 9 (range: 4-13) months. The median number of subsequent treatments was 1 (range: 1-4) and 7 patients had disease refractory to treatment. The median overall survival duration for STRs has not been reached: 11 (42%) have died, 7 because of CLL progression.
To identify features predictive for LTR, we compared pre-treatment clinical characteristics and baseline cytokines levels of the LTRs to those of the STRs. Median time from diagnosis to therapy was 51 (36-66) months, with no significant differences between STRs and LTRs (39 vs 54 months; P = .89). We observed that the LTRs had lower baseline β-2-microglobulin levels (P = .005) and were more likely to have trisomy 12 (0.03) and less likely to have deletion 17p (P = .005) (Table 1). Also, the baseline plasma levels of IL-8, interferon-γ, soluble vascular endothelial growth factor receptor-2, and chemokine (C-C motif) ligand 3 were significantly lower in the LTRs (P = .05; P = .006; P = .05; P = .002) than in the STRs, potentially indicating a lower level of T-cell dysfunction and activation of microenvironment stimulating pathways.
Patient characteristics and comparison between long-term and short-term responders
| . | Median (range), number (percentage) . | P . | |
|---|---|---|---|
| LTR (35 points) . | STR (25 points) . | ||
| Age (years) | 71 (66-85) | 71 (66-83) | .94 |
| Sex | |||
| Males | 19 (53%) | 15 (62%) | .50 |
| Females | 16 (47%) | 10 (38%) | |
| Rai stage | |||
| 0-II | 24 (71%) | 18 (69%) | .91 |
| III-IV | 11 (29%) | 7 (31%) | |
| Hemoglobin (g/dL) | 12 (9-16) | 12 (9-15) | 1.00 |
| Platelets (109/mL) | 140 (63-353) | 175 (82-464) | .30 |
| Lymphocytes (109/mL) | 49 (3-230) | 86 (2-222) | .12 |
| β-2-microglobulin (mg/L) | 3.9 (2-8) | 4.8 (2.5-10.2) | .005 |
| IgG (mg/dL) | 729 (180-3560) | 769 (273-2350) | .60 |
| CD3 (103/mL) | 3900 (642-11 822) | 3783 (875-42 750) | .60 |
| BM lymphocytes (%) | 76 (18-94) | 85 (36-96) | .11 |
| IGHV | |||
| Mutated | 11 (33%) | 11 (48%) | .27 |
| Unmutated | 23 (67%) | 11 (52%) | |
| ZAP70 | |||
| Positive | 20 (58%) | 14 (58%) | .99 |
| Negative | 14 (42%) | 11 (42%) | |
| CD38 | |||
| >30% | 17 (47%) | 14 (58%) | .41 |
| <30% | 18 (53%) | 11 (42%) | |
| FISH | |||
| Deletion 13 | 11 (32%) | 4 (15%) | .23 |
| Negative | 6 (18%) | 6 (23%) | .60 |
| Trisomy 12 | 11 (32%) | 2 (8%) | .03 |
| Deletion 11 | 7 (18%) | 7 (31%) | .23 |
| Deletion 17 | 0 (0%) | 6 (23%) | .005 |
| . | Median (range), number (percentage) . | P . | |
|---|---|---|---|
| LTR (35 points) . | STR (25 points) . | ||
| Age (years) | 71 (66-85) | 71 (66-83) | .94 |
| Sex | |||
| Males | 19 (53%) | 15 (62%) | .50 |
| Females | 16 (47%) | 10 (38%) | |
| Rai stage | |||
| 0-II | 24 (71%) | 18 (69%) | .91 |
| III-IV | 11 (29%) | 7 (31%) | |
| Hemoglobin (g/dL) | 12 (9-16) | 12 (9-15) | 1.00 |
| Platelets (109/mL) | 140 (63-353) | 175 (82-464) | .30 |
| Lymphocytes (109/mL) | 49 (3-230) | 86 (2-222) | .12 |
| β-2-microglobulin (mg/L) | 3.9 (2-8) | 4.8 (2.5-10.2) | .005 |
| IgG (mg/dL) | 729 (180-3560) | 769 (273-2350) | .60 |
| CD3 (103/mL) | 3900 (642-11 822) | 3783 (875-42 750) | .60 |
| BM lymphocytes (%) | 76 (18-94) | 85 (36-96) | .11 |
| IGHV | |||
| Mutated | 11 (33%) | 11 (48%) | .27 |
| Unmutated | 23 (67%) | 11 (52%) | |
| ZAP70 | |||
| Positive | 20 (58%) | 14 (58%) | .99 |
| Negative | 14 (42%) | 11 (42%) | |
| CD38 | |||
| >30% | 17 (47%) | 14 (58%) | .41 |
| <30% | 18 (53%) | 11 (42%) | |
| FISH | |||
| Deletion 13 | 11 (32%) | 4 (15%) | .23 |
| Negative | 6 (18%) | 6 (23%) | .60 |
| Trisomy 12 | 11 (32%) | 2 (8%) | .03 |
| Deletion 11 | 7 (18%) | 7 (31%) | .23 |
| Deletion 17 | 0 (0%) | 6 (23%) | .005 |
BM, bone marrow; FISH, fluorescence in situ hybridization; IGHV, immunoglobulin variable heavy chain.
In conclusion, our experience indicates that long-term treatment with lenalidomide-induced responses lasting more than 3 years in 58% of the patients. In a population of similar age, results with single agent bendamustine, chlorambucil, or fludarabine have been published. With the limitations of extrapolate results from individual trials and data limited to 2-year overall survival in these reports, the overall survival in this study compares favorably to the historical experience.15,16 We observed that the quality of responses continued to improve beyond 18 months and long-term treatment was well-tolerated. Recent reports have suggested a possible correlation between treatment with lenalidomide and the development of second cancers in patients with multiple myeloma.17 In our series only 1 patient developed an invasive skin cancer, but the size of this study and the available follow-up time are insufficient to derive accurate information on the occurrence of other cancers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.F. designed, performed, and analyzed the trial; A.F., X.C.B., M.J.K., W.G.W., S.O., H.M.K., and S.M.K. provided clinical care to patients; X.C.B. collected data; X.C.B., M.J.K., W.G.W., S.O., H.M.K., S.M.K., H.G., S.C., and J.M.R. coauthored the paper; P.S. analyzed data and performed statistical analysis; M.J.K. codesigned the trial; H.G., S.C. and J.R. analyzed laboratory data; and A.F. and P.S. wrote the paper.
Conflict-of-interest disclosure: M.J.K. and S.O. were consultants for Celgene Corporation; W.G.W. was a consultant/advisory board member for Celgene Corporation; and A.F. received research support from Celgene Corporation. The remaining authors declare no competing financial interests.
The current affiliation for X.C.B. is Department of Haematology, St. George Public Hospital, Kogarah, NSW, Australia.
Correspondence: Alessandra Ferrajoli, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: aferrajo@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal