In this issue of Blood, Moxon et al provide novel insight into the pathogenesis of cerebral malaria, linking loss of the endothelial protein C receptor (EPCR) on brain vessels, caused by cytoadherent infected erythrocytes, with localized coagulation, inflammation, and disruption of endothelial barrier function.1
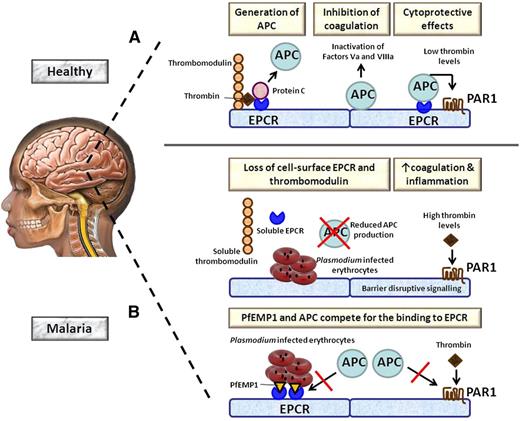
Role of EPCR in cerebral malaria. (A) During normal homeostasis, excessive thrombin is bound by thrombomodulin expressed by endothelial cells. The thrombomodulin-thrombin complex activates protein C to APC, a process that is strongly accelerated by EPCR. APC exerts anticoagulant effects when it becomes detached from EPCR by inactivating clotting factor Va and VIIIa. In addition, APC bound to EPCR has cytoprotective properties by activating PAR1. (B) (Upper) In malaria, parasite-infected erythrocytes induce the loss of EPCR and thrombomodulin from the endothelial cell surface at least in part by shedding of these receptors. As a consequence, the capacity to produce APC is greatly impaired, resulting in enhanced coagulation. The resulting high thrombin levels can induce proinflammatory barrier disruptive effects on blood vessels via PAR1. (Lower) Plasmodium-infected erythrocytes transport PfEMP1 to their membrane, which can bind EPCR in the same region as APC. As a result, APC is less capable of inducing cytoprotective effects via PAR1.
Role of EPCR in cerebral malaria. (A) During normal homeostasis, excessive thrombin is bound by thrombomodulin expressed by endothelial cells. The thrombomodulin-thrombin complex activates protein C to APC, a process that is strongly accelerated by EPCR. APC exerts anticoagulant effects when it becomes detached from EPCR by inactivating clotting factor Va and VIIIa. In addition, APC bound to EPCR has cytoprotective properties by activating PAR1. (B) (Upper) In malaria, parasite-infected erythrocytes induce the loss of EPCR and thrombomodulin from the endothelial cell surface at least in part by shedding of these receptors. As a consequence, the capacity to produce APC is greatly impaired, resulting in enhanced coagulation. The resulting high thrombin levels can induce proinflammatory barrier disruptive effects on blood vessels via PAR1. (Lower) Plasmodium-infected erythrocytes transport PfEMP1 to their membrane, which can bind EPCR in the same region as APC. As a result, APC is less capable of inducing cytoprotective effects via PAR1.
Malaria is caused by parasites of the genus Plasmodium, of which P falciparum is the most virulent.2 One of the most fatal manifestations of P falciparum infection is cerebral malaria, which especially affects children <6 years of age and is responsible for an annual death toll of nearly a million infants in Africa alone. In recent years, the cycle of coagulation and inflammation has emerged as a pivotal component of malaria pathogenesis.3 In normal homeostasis, activation of coagulation is compensated by concurrent induction of anticoagulant mechanisms.4 In areas of excessive activation of the clotting cascade, thrombin acts as a feedback inhibitor of coagulation by binding to the endothelial receptor thrombomodulin (see figure). The thrombomodulin-thrombin complex converts the zymogen protein C into activated protein C (APC), a reaction that is greatly accelerated by EPCR.5,6 APC acts as an anticoagulant by virtue of its capacity to proteolytically inactivate clotting factors Va and VIIIa. When attached to EPCR, APC in addition can exert anti-inflammatory, antiapoptotic, and vasculoprotective signals via protease activated receptor (PAR)1. In case of impaired function of the protein C system, high thrombin levels can activate PAR1, resulting in effects that are opposite to those transduced by APC, disrupting endothelial barrier function.
A hallmark feature of severe malaria is sequestration of infected erythrocytes in blood vessels.2 The elegant investigations by Moxon et al provide a long-sought explanation of why red blood cell sequestration especially leads to damage in the brain.1 In postmortem studies in children that had died of cerebral malaria, endothelial sites of adherent erythrocytes were shown to colocalize with loss of EPCR. Moreover, the authors developed a novel approach that enabled examination of blood vessels with relevance for the brain vasculature in children with cerebral malaria directly after admission to the hospital. For this, they used subcutaneous tissue microvessels as an ex vivo surrogate for brain endothelium and demonstrated reduced expression of both EPCR and thrombomodulin in cerebral malaria patients compared with healthy controls. Children with cerebral malaria had higher levels of soluble EPCR and thrombomodulin in their cerebrospinal fluid than febrile control patients, suggesting that the loss of these receptors at least in part was caused by shedding. Importantly, in plasma, the balance between the production of thrombin (measured by the levels of the prothrombin F1+2 fragment) and APC was not altered in cerebral malaria compared with healthy children and children with mild febrile disease or uncomplicated malaria, indicating that at the systemic level, coagulation activation was compensated. Together, these data strongly suggest that cerebral malaria is associated with a localized disturbance of coagulation and inflammation caused by a local loss of EPCR and thrombomodulin initiated by sequestration of infected erythrocytes, changes that damage the brain due to the already low constitutive expression of EPCR and thrombomodulin in healthy brain vessels.1,7
Another recent study has implicated EPCR in the pathogenesis of malaria by a completely different mechanism.8 In malaria, adherence of red blood cells to endothelium is caused by an interaction between P falciparum erythrocyte membrane protein-1 (PfEMP1) family members, transported to the erythrocyte membrane as a consequence of parasite infection, and receptors on the vascular endothelium.2 In this respect, PfEMP1 subtypes containing domain cassettes 8 and 13 are important for sequestration of infected erythrocytes in severe childhood malaria.9 Turner et al very recently revealed EPCR as the endothelial receptor for PfEMP1 domain cassettes 8 and 13.8 In addition, these authors demonstrated that parasites causing severe malaria exhibited stronger EPCR binding than parasites from children with uncomplicated malaria. Of considerable interest and importance, PfEMP1 was shown to bind EPCR near or at the same region of APC, implicating that EPCR-mediated cytoadhesion likely inhibits APC-mediated EPCR-dependent cytoprotective effects on endothelial cells.8
These studies by Moxon et al1 and Turner et al8 not only expose EPCR as a critical player in malaria, but they also provide the rationale for novel therapeutic strategies. Administration of recombinant human APC is expected to (partially) restore the functional APC deficiency imposed by loss of EPCR expression and at the same time to hinder adhesion of infected erythrocytes to endothelium by inhibiting the interaction between EPCR and PfEMP1. The question that remains is whether the anticoagulant effects of APC would be required for these anticipated beneficial effects in malaria. The study by Moxon et al1 leaves this question unanswered, although drawing a parallel with bacterial sepsis and cerebrovascular brain damage, it seems likely that the cytoprotective PAR1-mediated effects of APC are important for preventing brain injury in malaria.5,10 In addition, although not directly examined by Turner et al,8 it is to be expected that APC’s anticoagulant properties are not important for interfering with the binding of PfEMP1 to EPCR. These results therefore set the stage for therapeutic studies in malaria with recombinant human APC mutants that lack anticoagulant properties but with preserved cytoprotective capacity. Such selective APC mutants have been developed and bear the important advantage that they do not expose patients to the risk of bleeding complications.5 One of these APC mutants (3K3A-APC) has reached the phase of safety and pharmacokinetic studies in healthy humans (clinicaltrials.gov identifier NCT01660230).
Conflict of interest disclosure: The author declares no competing financial interests.