Key Points
BMP9 is required for lymphatic valve formation.
Mice deficient in Bmp9 exhibit reduction in lymphatic draining efficiency.
Abstract
Lymphatic vessels are critical for the maintenance of tissue fluid homeostasis and their dysfunction contributes to several human diseases. The activin receptor-like kinase 1 (ALK1) is a transforming growth factor-β family type 1 receptor that is expressed on both blood and lymphatic endothelial cells (LECs). Its high-affinity ligand, bone morphogenetic protein 9 (BMP9), has been shown to be critical for retinal angiogenesis. The aim of this work was to investigate whether BMP9 could play a role in lymphatic development. We found that Bmp9 deficiency in mice causes abnormal lymphatic development. Bmp9-knockout (KO) pups presented hyperplastic mesenteric collecting vessels that maintained LYVE-1 expression. In accordance with this result, we found that BMP9 inhibited LYVE-1 expression in LECs in an ALK1-dependent manner. Bmp9-KO pups also presented a significant reduction in the number and in the maturation of mesenteric lymphatic valves at embryonic day 18.5 and at postnatal days 0 and 4. Interestingly, the expression of several genes known to be involved in valve formation (Foxc2, Connexin37, EphrinB2, and Neuropilin1) was upregulated by BMP9 in LECS. Finally, we demonstrated that Bmp9-KO neonates and adult mice had decreased lymphatic draining efficiency. These data identify BMP9 as an important extracellular regulator in the maturation of the lymphatic vascular network affecting valve development and lymphatic vessel function.
Introduction
The lymphatic vasculature is essential for the maintenance of normal fluid balance and for the immune response. It consists of a network of vessels that drain protein-rich lymph from the extracellular space back to the blood circulation, which absorbs dietary fatty acids and is involved in the traffic of immune cells.1 Lymphatic vessels may also serve as a conduit to lymph nodes and thereby participate in systemic metastasis of cancer cells. Hypoplasia, disruption or dysfunction of the lymphatic vessels, impair the ability of the lymphatic vasculature to collect and transport fluids and lead to lymphedema.1,2
The mammalian lymphatic system has been shown to originate from embryonic veins. Lymphatic vessel development starts at embryonic days (E) 9.5 to 10.5 in mice. After formation of the primary lymph sacs, further expansion leads to the formation of a primary lymphatic vascular plexus that, through subsequent remodeling and maturation, will provide a hierarchical network of lymphatic capillaries and lymphatic collecting vessels.1,3 During this vessel specification, maturation of collecting vessels is accompanied by the downregulation of lymphatic marker molecules such as LYVE-1, the acquisition of partial smooth muscle cell coverage, and the formation of intraluminal valves.4-6 Lymphatic valves are essential components that ensure unidirectional lymph flow. They develop from E15.5 to early postnatal days in mice.6 Few key molecular regulators of their formation and/or their maturation have been identified. It was shown that the mechanical stimulus caused by lymph flow establishes the location where valves develop by upregulating the expression of Foxc2. Foxc2 cooperates with Prox1 to control Connexin37 (Cx37) expression and to activate NFATc1/Calcineurin signaling.7,8 Other molecular regulators such as EphrinB2, integrin α9, Connexin43 (Cx43), Semaphorin3a, and Neuropilin1 (Nrp-1) have been reported to be important for either lymphatic valve formation, elongation of lymphatic valve leaflets, or valve maturation.5,8-12 However, still not much is known concerning the extracellular factors that govern valve development.
The activin receptor-like kinase 1 (ALK1) is a type 1 receptor of the transforming growth factor-β family that is specifically expressed on endothelial cells.13 We have previously shown that bone morphogenetic protein 9 (BMP9) and BMP10 bind with high affinity to ALK1.14 BMP9 and BMP10 have been recently shown to control postnatal retinal vascularization.15 Interestingly, ALK1 is expressed on both blood endothelial cells and lymphatic endothelial cells (LECs) and it was recently shown that LECs respond to BMP9 and BMP10 by inducing Smad6 expression; this effect is mediated by ALK1.16 It was further shown that treatment of neonatal mice between postnatal day 1 (P1) and P3 with the ALK1 extracellular domain (ALK1Fc) impaired the development of the honeycomb pattern of lymphatic vessels of the tail dermis and of the intestinal villi.16 Therefore, these data indicated a role for ALK1 ligands in the formation of lymphatic capillaries at early postnatal stages. We have reported that BMP9 is present in plasma and that its circulating level is elevated in mouse embryos just before birth and during early postnatal life,14,15,17 consistent with a potential role for BMP9 in the lymphatic system maturation that occurs during this period.
To investigate the role of BMP9 on lymphatic maturation and valve formation, we used Bmp9-knockout (KO) mice, which are viable and do not present any obvious phenotype.15 We report here that Bmp9 deficiency leads to defects in both lymphatic capillaries and collecting vessel maturation and in valve formation. This abnormal lymphatic vascular patterning resulted in a decrease in draining efficiency both in neonates and in adult mice. Several previously identified genes and proteins involved in lymphatic vessel maturation and valve formation were identified as molecular targets of BMP9 (LYVE-1, Foxc2, Cx37, EphrinB2, and Nrp-1). Taken together, these data provide evidence for a critical role of BMP9 in the development of lymphatic vessels.
Methods
Mice
All animal studies were approved by the institutional guidelines and those formulated by the European Community for the Use of Experimental Animals. The generation of Bmp9-KO mice and genotyping were described previously.15 Mice were maintained on a C57BL/6 genetic background.
Whole-mount immunofluorescent stainings
Mesenteries and skins were dissected and attached with pins on elastomer-coated plates and fixed overnight with 4% paraformaldehyde diluted in phosphate-buffered saline (PBS). Whole-mount immunofluorescent stainings were performed essentially as previously described.7,18 After washes with PBS, tissues were blocked overnight with PBS containing 2% bovine serum albumin and 0.3% Triton X-100. They were then incubated with primary antibodies overnight at 4°C. Primary antibodies used are listed in the supplemental Methods. Alexa fluor 488- or Cyanin-3–conjugated affinity-purified minimal crossreactivity secondary antibodies (Jackson ImmunoResearch) were used for the staining. After extensive washes, samples were postfixed in 4% paraformaldehyde and mounted with Fluorsave reagent (Calbiochem). The images were acquired and analyzed using either a ZEISS Axio-observer Z1 inverted fluorescence microscope and Axiovision 4.8 software or a LEICA scanning confocal microscope (TCS SP2). All images were processed with Adobe Photoshop CS5 software.
LEC culture, treatment, and siRNA transfection
Human dermal LECs were obtained from LONZA Biosciences and cultured in EGM-2 medium containing endothelial cell growth supplements with 5% fetal bovine serum. They were used between passage 5 and 7. For small interfering RNA (siRNA) experiments, LECs were transfected with 10 nM siRNA using RNAimax (Life Technologies) according to the manufacturer’s instructions. All siRNAs were purchased from Ambion (Alk1, #s986, and siRNA scramble, AM4613). Cells were stimulated in serum-free medium with 10 ng/mL recombinant human BMP9 (R&D Systems) at the times indicated.
Quantitative real-time PCR
Total RNAs were extracted at the indicated times using the Nucleospin RNA XS kit (Macherey-Nagel). First-strand cDNAs were synthesized from 1 µg total RNA by reverse transcription using the iScript system (Bio-Rad) according to the manufacturer’s instructions. Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using a Bio-Rad CFX96 apparatus and qPCR Master Mix (Promega). Relative quantification of gene expression was normalized to hypoxanthine phosphoribosyltransferase (HPRT) messenger RNA (mRNA) expression level. Sequences of the PCR primers used are listed in supplemental Table 1.
Flow cytometry analysis
For flow cytometry analysis, cells were detached by incubation for 30 minutes at 37°C in nonenzymatic cell dissociation buffer. After 2 washes in PBS, cells were stained with LYVE-1 antibodies in PBS containing 2% bovine serum albumin for 1 hour at 4°C. The binding of the primary antibody was revealed by incubation with Alexa fluor 488–conjugated secondary antibodies. Cells were analyzed on a FacsCalibur flow cytometer (BD Biosciences) using CellQuest software (BD Biosciences).
Lymphatic vessel tracing and analysis of lymphatic vessel function
The functionality of the lymphatic network in adult mice was investigated by noninvasive real-time fluorescence imaging of lymphatic function assessed after injection of hydrophobic cyanine dye–loaded nanoparticles (DiD-lipidots) of 50 nm diameter into the hind limb footpads of anesthetized adult mice. These neutral dye–loaded oily nanodroplets (with narrow size distribution) dispersed in aqueous buffer have been shown to display high brightness suitable for in vivo fluorescence imaging.19,20 Moreover, because of their very small size (<100 nm) and their lipid nature, these particles are relevant not only for efficient lymphatic draining through lymph vessels, but also for their partial retention into lymph nodes.20-22 DiD-lipidots’ excitation and emission maxima were 646 and 668 nm, respectively. The DiD-lipidots’ dispersion was prepared as previously described.19 After injection of 5 µL of 10-µM dye, the mouse was placed inside a whole body in vivo imaging system (IVIS; Caliper Life Sciences). The uptake of DiD-lipidots by dermal lymphatic capillaries that feed lymphatic precollector and collector vessels was followed over a 15-minute period. Image acquisition and analysis were performed with Living Image 3 software (Caliper Life Sciences) using the cyanine5.5 fluorescence emission filter.
Statistical analysis
Statistical data analysis was assessed using either unpaired Student t test or Mann-Whitney U test.
Results
Bmp9 deficiency affects the development of lymphatic collecting vessels of the mesentery
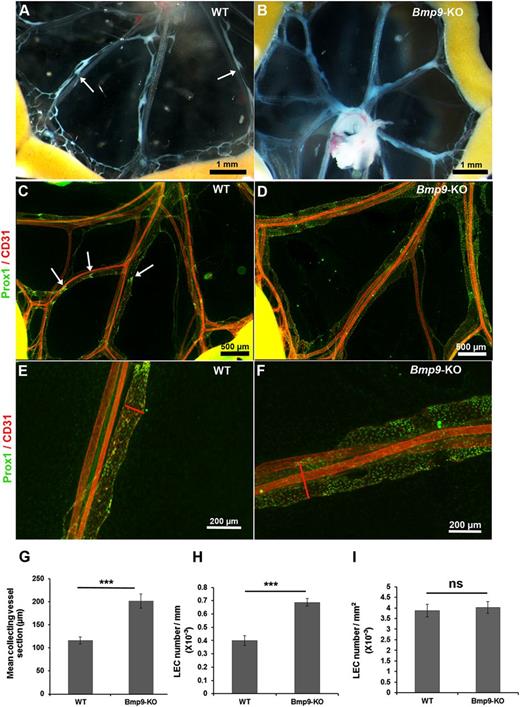
To investigate a potential involvement of BMP9 during lymphatic collecting vessel maturation, we examined mesenteries of wild-type (WT) and Bmp9-KO mice at P0. The analysis of chyle-containing mesenteric collecting vessels in WT neonates revealed characteristic constrictions reflecting functional valves (Figure 1A). On the other hand, Bmp9-KO neonates displayed morphologically enlarged lymphatic vessels and the constrictions corresponding to valve territories were less visible (Figure 1B). Visualization of the lymphatic vessels by immunofluorescence staining of the nuclear transcription factor Prox1, which is restricted to the lymphatic vessels; the pan-endothelial marker CD31 confirmed these observations (Figure 1C-D). Several areas of dense Prox1-overexpressing cells, a typical feature of lymphatic valves, were observed in the mesentery of WT pups but were missing in Bmp9-KO pups. Higher magnification views of the mid-part of representative collecting vessels confirmed lymphatic enlargement in Bmp9-KO neonates (Figure 1E-F). Quantitative analysis of the mean section of lymphatic collecting vessels confirmed the enlarged phenotype of these vessels in Bmp9-KO as compared with WT pups (Figure 1G). Interestingly, the number of LECs (identified as Prox1-positive cells) per collecting vessel unit length was significantly higher in Bmp9-KO than in WT, whereas the number of LECs per surface unit was not different, indicating that this enlargement resulted from cellular hyperplasia rather than cellular hypertrophy (Figure 1H-I). Lymphatic capillaries (identified by lymphatic-specific hyaluronan receptor LYVE-1–positive staining) of the ventral skin of P0 neonates were also found enlarged, with some areas of hyperdilated and sac-like disorganized lymphatic vessels in Bmp9-KO as compared with WT newborns (supplemental Figure 1, left panel). On the other hand, the sprouting of lymphatic capillaries from the dorsal skin at E15.5 was not significantly different in Bmp9-KO as compared with WT mice (supplemental Figure 1, right panel). Interestingly, the blood vasculature of P0 Bmp9-KO neonates (ventral skin) was not affected (supplemental Figure 2).
Abnormal structure of lymphatic mesenteric collecting vessels in Bmp9-KO neonates at P0. Representative macroscopic views of mesenteric vessels in WT (A) and Bmp9-KO (B) pups at P0. The lymphatic vessels are filled with white chyle and constrictions along the vessels reflecting the presence of valves are shown with white arrows. Whole-mount fluorescence immunostainings of WT (C) and Bmp9-KO (D) mesenteries with CD31 (red) and Prox1 (green); arrows point to Prox1-overexpressing cells in valve areas. Higher magnification views of the mid-part of mesenteric collecting vessels in WT (E) or Bmp9-KO (F). Enlarged vessels, as outlined by the red lines, showing vessel sections are observed in mutants. (G) Quantification of the mean collecting vessel section in WT and Bmp9-KO pups. The mean diameter of mesenteric lymphatic collecting vessels was calculated as the average of 4 different measurements performed using Axiovision 4.8 software throughout the length of 4 different collecting vessels per mesentery. (H-I) Quantification of LEC number per millimeter of collecting vessel length and by square millimeter of collecting vessel surface unit in WT and Bmp9-KO pups. Prox1-positive nuclei were counted per vessel unit length and per vessel surface unit on at least 3 collecting vessel per mesentery using Axiovision 4.8 software. Values are the mean (± SE) from 8 individuals per genotype. ns, not significant; ***P ≤ .001, significantly different from WT pups by unpaired Student t test.
Abnormal structure of lymphatic mesenteric collecting vessels in Bmp9-KO neonates at P0. Representative macroscopic views of mesenteric vessels in WT (A) and Bmp9-KO (B) pups at P0. The lymphatic vessels are filled with white chyle and constrictions along the vessels reflecting the presence of valves are shown with white arrows. Whole-mount fluorescence immunostainings of WT (C) and Bmp9-KO (D) mesenteries with CD31 (red) and Prox1 (green); arrows point to Prox1-overexpressing cells in valve areas. Higher magnification views of the mid-part of mesenteric collecting vessels in WT (E) or Bmp9-KO (F). Enlarged vessels, as outlined by the red lines, showing vessel sections are observed in mutants. (G) Quantification of the mean collecting vessel section in WT and Bmp9-KO pups. The mean diameter of mesenteric lymphatic collecting vessels was calculated as the average of 4 different measurements performed using Axiovision 4.8 software throughout the length of 4 different collecting vessels per mesentery. (H-I) Quantification of LEC number per millimeter of collecting vessel length and by square millimeter of collecting vessel surface unit in WT and Bmp9-KO pups. Prox1-positive nuclei were counted per vessel unit length and per vessel surface unit on at least 3 collecting vessel per mesentery using Axiovision 4.8 software. Values are the mean (± SE) from 8 individuals per genotype. ns, not significant; ***P ≤ .001, significantly different from WT pups by unpaired Student t test.
BMP9 regulates LYVE-1 expression
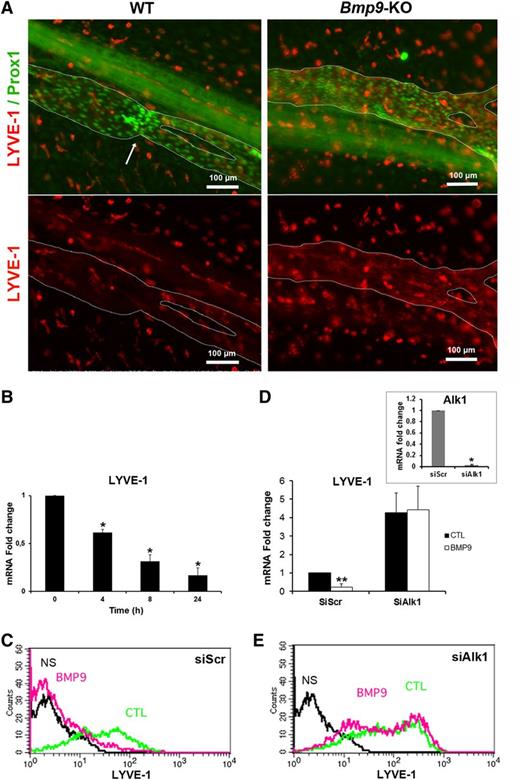
A loss of LYVE-1 expression is observed during the maturation of collecting vessels.5,6 Therefore, to explore whether the enlarged collecting vessels observed in Bmp9-KO pups could be due to abnormal maturation, P0 mesenteric collecting vessels were stained with LYVE-1 and Prox1. LYVE-1 expression was very low in WT-collecting vessels at P0. In contrast, there was still a detectable LYVE-1 immunoreactivity in Bmp9-KO pups, suggesting an impairment of lymphatic collecting vessel maturation in the absence of BMP9 (Figure 2A). We also found that recombinant BMP9 inhibited LYVE-1 expression in primary cultures of LECs at both its mRNA and protein levels (Figure 2B-C). We further demonstrated that this inhibition was mediated by ALK1 using an RNA interference strategy. When ALK1 was silenced, BMP9 was no longer able to downregulate LYVE-1 mRNA and protein expression in LECs (Figure 2D-E). Interestingly, and consistent with the BMP9-induced LYVE-1 downregulation, Alk1 silencing resulted in an increase in LYVE-1 expression. Targeted knockdown of ALK1 mRNA expression was verified by quantitative RT-PCR (Figure 2D, inset).
LYVE-1 expression is regulated by BMP9 both in vivo in lymphatic collecting vessels of the mesentery and in vitro in cultured LECs. (A) P0 lymphatic mesenteric collecting vessels were stained for LYVE-1 (red) and Prox1 (green). The dashed lines delineate the lymphatic vessels. Arrow indicates a valve. A nonlymphatic LYVE-1 immunoreactivity is also observed in isolated cells outside vessels, which probably correspond to macrophages. (B) Time-dependent inhibition of LYVE-1 mRNA expression in LECs treated with BMP9 (10 ng/mL); the results are presented as mRNA fold changes measured in BMP9-treated cells vs nontreated cells at each time point. Data are the mean ± SE from 6 independent experiments performed in duplicate. *P ≤ .05, significantly different from cytotoxic T lymphocyte (CTL) by Mann-Whitney U test. (C,E) Flow cytometry detection of LYVE-1 protein expression in LECs transfected for 24 hours with scramble siRNA or siRNA targeting Alk1 and then stimulated with (pink) or without (green) 10 ng/mL BMP9 for 48 hours. NS corresponds to fluorescence-activated cell sorter analysis in absence of antibodies (black). (D) LYVE-1 mRNA expression in LECs transfected for 24 hours with scrambled siRNA (siScr) or siRNA targeting Alk1 and then stimulated with or without 10 ng/mL BMP9 for 24 hours; data represent mean ± SE (n = 6). **P ≤ .01, significantly different from CTL by Mann-Whitney U test. Inset shows Alk1 mRNA downregulation by siAlk1 (mean ± SE, n = 4). *P ≤ .05, significantly different from siCTL by Mann-Whitney U test.
LYVE-1 expression is regulated by BMP9 both in vivo in lymphatic collecting vessels of the mesentery and in vitro in cultured LECs. (A) P0 lymphatic mesenteric collecting vessels were stained for LYVE-1 (red) and Prox1 (green). The dashed lines delineate the lymphatic vessels. Arrow indicates a valve. A nonlymphatic LYVE-1 immunoreactivity is also observed in isolated cells outside vessels, which probably correspond to macrophages. (B) Time-dependent inhibition of LYVE-1 mRNA expression in LECs treated with BMP9 (10 ng/mL); the results are presented as mRNA fold changes measured in BMP9-treated cells vs nontreated cells at each time point. Data are the mean ± SE from 6 independent experiments performed in duplicate. *P ≤ .05, significantly different from cytotoxic T lymphocyte (CTL) by Mann-Whitney U test. (C,E) Flow cytometry detection of LYVE-1 protein expression in LECs transfected for 24 hours with scramble siRNA or siRNA targeting Alk1 and then stimulated with (pink) or without (green) 10 ng/mL BMP9 for 48 hours. NS corresponds to fluorescence-activated cell sorter analysis in absence of antibodies (black). (D) LYVE-1 mRNA expression in LECs transfected for 24 hours with scrambled siRNA (siScr) or siRNA targeting Alk1 and then stimulated with or without 10 ng/mL BMP9 for 24 hours; data represent mean ± SE (n = 6). **P ≤ .01, significantly different from CTL by Mann-Whitney U test. Inset shows Alk1 mRNA downregulation by siAlk1 (mean ± SE, n = 4). *P ≤ .05, significantly different from siCTL by Mann-Whitney U test.
BMP9 is required for lymphatic valve formation in mesenteric collecting vessels
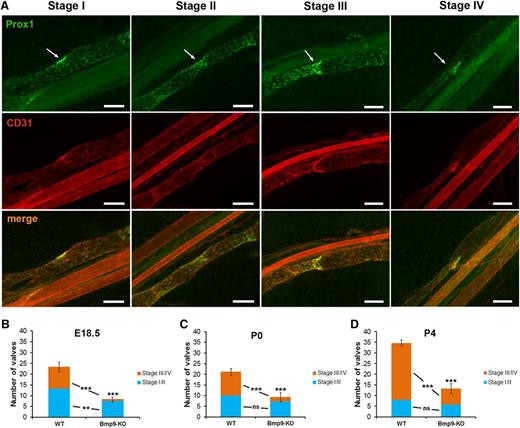
An important feature in lymphatic collecting vessel development and maturation is the formation of valves.23 Four different stages of valve development have been previously defined.7,8 Figure 3A illustrates these valve maturation stages on the basis of Prox1 and CD31 costaining. Stage I is marked by the appearance of cell clusters expressing high levels of the transcription factor Prox1 at sites of developing valves. The establishment of the valve territory by coalescence of Prox1-overexpressing cells by a circularization process defines stage II. The formation of a ring-like constriction concomitant with the initiation of the formation of valve leaflets constitutes stage III, whereas stage IV is characterized by the elongation of valve leaflets into the lumen of the vessel and leads to the formation of a mature V-shaped valve. We quantified mesenteric lymphatic valves in both WT and Bmp9-KO mice and classified them into 2 categories: immature (stage I/II) and mature (stage III/IV). We observed a significant reduction in the total number of valves in Bmp9-KO mutants at E18.5 as compared with WT (Figure 3B). This decrease was significant both in the number of immature and mature valves. A highly significant reduction in the total number of valves was also observed at P0 and P4 (Figure 3C-D). However, this difference was mainly due to a decrease in the number of mature valves. Still, the mature valves observed in P4 Bmp9-KO pups were correctly specified as assessed by the presence of the transcription factor Foxc2 and of the cell matrix adhesion receptor integrin α923 and by the absence of smooth muscle cell coverage of valve regions24 (supplemental Figure 3).
Defective lymphatic valve formation in Bmp9-KO embryos and neonates. (A) WT P0 lymphatic mesenteric collecting vessels were stained for Prox1 (green) and CD31 (red) to allow the discrimination between the different valve maturation stages; arrows point to the valve location; bars, 100 µm. (B-D) Quantitative analysis of valve formation at E18.5, P0, and P4 in WT and Bmp9-KO mice. Values correspond to the number of valves counted on 4 collecting vessels per mesentery; n = 6 (Bmp9-KO) or n = 7 (WT) at E18.5; n = 8 (Bmp9-KO) or n = 9 (WT) at P0; n = 12 (Bmp9-KO) or n = 11 (WT) at P4. ns, not significant. **P ≤ .01, ***P ≤ .001, significantly different from WT pups by unpaired Student t test.
Defective lymphatic valve formation in Bmp9-KO embryos and neonates. (A) WT P0 lymphatic mesenteric collecting vessels were stained for Prox1 (green) and CD31 (red) to allow the discrimination between the different valve maturation stages; arrows point to the valve location; bars, 100 µm. (B-D) Quantitative analysis of valve formation at E18.5, P0, and P4 in WT and Bmp9-KO mice. Values correspond to the number of valves counted on 4 collecting vessels per mesentery; n = 6 (Bmp9-KO) or n = 7 (WT) at E18.5; n = 8 (Bmp9-KO) or n = 9 (WT) at P0; n = 12 (Bmp9-KO) or n = 11 (WT) at P4. ns, not significant. **P ≤ .01, ***P ≤ .001, significantly different from WT pups by unpaired Student t test.
BMP9 regulates the expression of several master genes known to be involved in valve formation
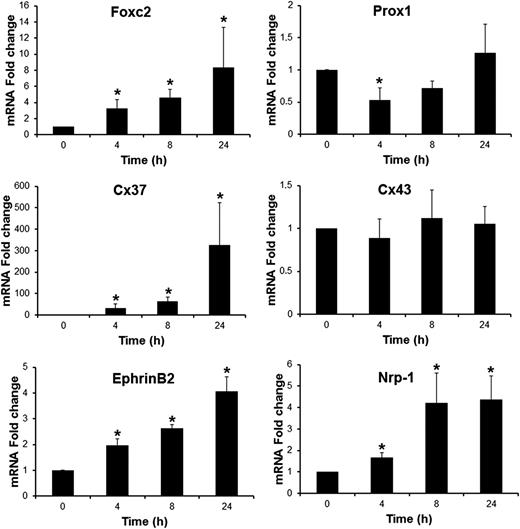
Several key molecular regulators associated with valve formation and their different stages of maturation have been identified.8,23 We therefore analyzed whether these genes could constitute molecular targets for BMP9 in LECs by measuring their expression by quantitative real-time RT-PCR. BMP9 increased the mRNA levels of Foxc2, Cx37, EphrinB2, and Nrp-1 in a time-dependent manner, whereas no significant variations were detected in NFATc1, integrinα9, Cx43, and Sema3a levels (Figure 4 and data not shown). The Foxc2 mRNA level was increased to eightfold 24 hours after BMP9 treatment, whereas EphrinB2 and Nrp-1 were increased around fourfold. The Cx37 mRNA level was hardly detectable in LECs without BMP9 treatment and was very potently induced in response to BMP9 (more than 300-fold). As observed for LYVE-1 expression, BMP9 regulated the expression of these genes in an Alk1-dependent manner (data not shown). We also observed a small transient but significant inhibition in Prox1 expression (50% reduction in mRNA level after 4 hours of BMP9 treatment).
BMP9-regulated genes, known to be involved in lymphatic valve development, in LECs. LECs were stimulated in serum-free medium in the absence or in the presence of 10 ng/mL BMP9 for the indicated times. Expression of HPRT was used to normalize the samples. The results are represented as mRNA fold changes measured in BMP9-treated cells vs nontreated cells at each time point. Data are the mean ± SE from 4 independent experiments performed in duplicate. *P ≤ .05, significantly different from respective controls by Mann-Whitney U test.
BMP9-regulated genes, known to be involved in lymphatic valve development, in LECs. LECs were stimulated in serum-free medium in the absence or in the presence of 10 ng/mL BMP9 for the indicated times. Expression of HPRT was used to normalize the samples. The results are represented as mRNA fold changes measured in BMP9-treated cells vs nontreated cells at each time point. Data are the mean ± SE from 4 independent experiments performed in duplicate. *P ≤ .05, significantly different from respective controls by Mann-Whitney U test.
BMP9 deficiency causes an abnormal lymphatic vasculature patterning and a decrease in lymphatic draining efficiency in adult mice
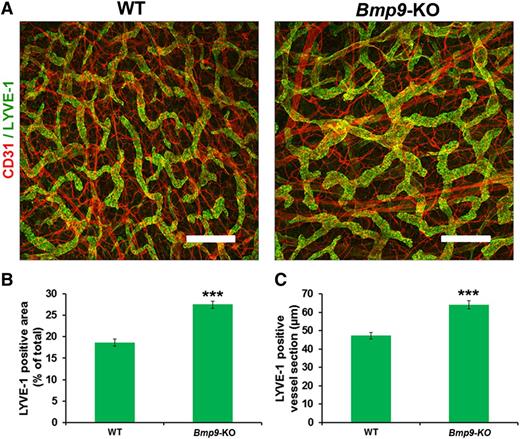
We then investigated whether Bmp9 deficiency also affects the growth and patterning of the lymphatic vessels in adult mice. Adult mouse ear skin vessels were stained with LYVE-1 and CD31. As shown in Figure 5A, the lymphatic capillary vessels formed a network of mostly small and often blind-end capillaries in WT mice, whereas enlarged or dilated cutaneous lymphatic vessels were seen in Bmp9-KO ears. Morphometric analyses confirmed that the average area of LYVE-1–positive cells and the mean section of LYVE-1–positive capillary vessels were significantly increased in Bmp9-KO mice vs WT (Figure 5B-C). Moreover, the defect in valve formation was not restricted to the mesentery and still persisted in the adult, as assessed by the observation of a significant reduction in valve number in the diaphragm of adult Bmp9-KO mice (supplemental Figure 4). Similarly to our observations in neonates, no significant defects were observed in blood vessels (ear) in adult Bmp9-KO mice (supplemental Figure 2).
Abnormal patterning of ear lymphatic capillaries in adult Bmp9-KO mice. (A) Adult ear lymphatic capillaries were stained for LYVE-1 (green) and CD31 (red). Note that the LYVE-1 staining overlies weak CD31 staining in the lymphatic vessels. Bar represents 300 µm. (B) Quantification of the mean LYVE-1–positive area expressed as percentage of total area. The lymphatic vessel area in the inner layer of the ear of adult mice was measured using Image J software on images corresponding to 3 different fields of whole ear skin. These regions were kept constant for all samples. (C) Quantification of the mean LYVE-1–positive lymphatic vessel section expressed in micrometers. To quantify ear skin capillary mean lymphatic size, 5 horizontal lines were evenly laid on the images, and the diameters of lymphatic vessels that crossed these lines with an angle above 45° were measured using Image J according to Zhou et al.31 Values are mean ± SE; n = 8 for each genotype. ***P ≤ .001, significantly different from WT pups by unpaired Student t test.
Abnormal patterning of ear lymphatic capillaries in adult Bmp9-KO mice. (A) Adult ear lymphatic capillaries were stained for LYVE-1 (green) and CD31 (red). Note that the LYVE-1 staining overlies weak CD31 staining in the lymphatic vessels. Bar represents 300 µm. (B) Quantification of the mean LYVE-1–positive area expressed as percentage of total area. The lymphatic vessel area in the inner layer of the ear of adult mice was measured using Image J software on images corresponding to 3 different fields of whole ear skin. These regions were kept constant for all samples. (C) Quantification of the mean LYVE-1–positive lymphatic vessel section expressed in micrometers. To quantify ear skin capillary mean lymphatic size, 5 horizontal lines were evenly laid on the images, and the diameters of lymphatic vessels that crossed these lines with an angle above 45° were measured using Image J according to Zhou et al.31 Values are mean ± SE; n = 8 for each genotype. ***P ≤ .001, significantly different from WT pups by unpaired Student t test.
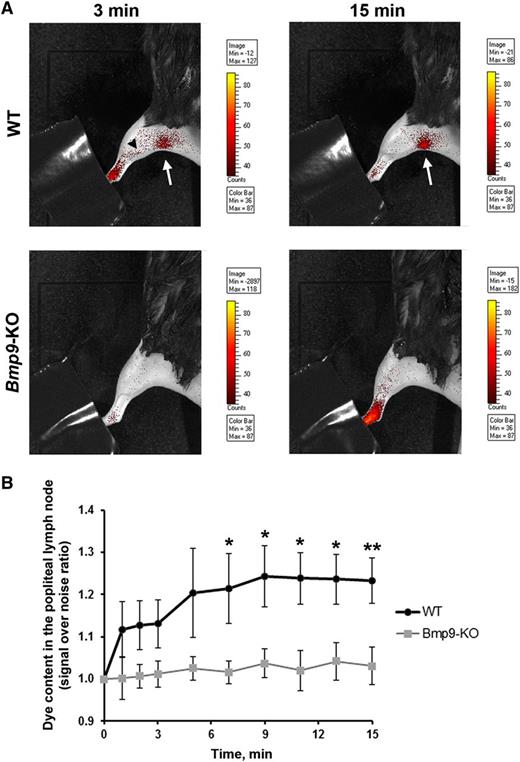
To investigate whether Bmp9-KO mice may have defects in their functional draining response, we performed a noninvasive real-time analysis of draining efficiency after injection of fluorescent nanoparticles (DiD-lipidots)19,20 in the hind limb footpad of adult mice. We observed that in WT animals the dye was rapidly drained by lymphatic vessels and reached, as early as 3 minutes after injection, the popliteal lymph node (6 limbs over 9 tested, Figure 6A). In contrast, in Bmp9-KO mice, dye accumulation into the popliteal lymph node failed to be detected without skinning of the animals (6 limbs over 6 tested, Figure 6A). In Bmp9-KO mice, the uptake of the dye remained mostly diffuse in the limb and did not label the deep collectors as in WT mice (Figure 6A). Measurements of the kinetic of dye accumulation into the popliteal lymph node region revealed a significant difference between WT and Bmp9-KO mice from 7 minutes after injection (Figure 6B). These defects did not result from alteration in lymph nodes and/or collecting vessels structures as assessed by their apparent normal morphological features after Evans blue injection (supplemental Figure 5). The lymphatic function was also evaluated in P4 neonates 15 minutes after injection of fluorescein isothiocyanate–dextran into the hind limb footpad. After sacrifice and skin removal, we observed that the dye was drained into the collecting vessels leading to the popliteal lymph node in both WT and Bmp9-KO pups (supplemental Figure 6A-B). However, when examining the superficial lymphatic vascular network of the skin recovering these tissues, we observed that fluorescein isothiocyanate–dextran-labeled vessels were rarely found in WT pups (supplemental Figure 6C,E), whereas in contrast a network of labeled capillaries was detected in most Bmp9-KO pups (supplemental Figure 6D,F) suggesting a less efficient draining capacity in these pups as in the adults. In addition, these vessels often exhibited enlarged areas that confirmed our previous observations performed in the ventral skin of P0 pups (supplemental Figure 1).
Impairment of lymphatic drainage in adult Bmp9-KO mice. (A) Representative fluorescence images of WT and Bmp9-KO hind limbs obtained 3 and 15 minutes after injection of DiD-lipidots. The extremity of the paw was hidden not to saturate the images by the fluorescence signal at the injection site. The black arrowhead outlines lymphatic collectors; the white arrow indicates the popliteal lymph node. The values of the scale bars for fluorescence intensity were adjusted to normalize each image series at comparable values. (B) Quantitative analysis of dye accumulation into the popliteal lymph node. Signal over noise ratio was measured. Values are means ± SE; n = 6 (Bmp9-KO) or n = 9 (WT). *P ≤ .05, significantly different by Mann-Whitney U statistical test.
Impairment of lymphatic drainage in adult Bmp9-KO mice. (A) Representative fluorescence images of WT and Bmp9-KO hind limbs obtained 3 and 15 minutes after injection of DiD-lipidots. The extremity of the paw was hidden not to saturate the images by the fluorescence signal at the injection site. The black arrowhead outlines lymphatic collectors; the white arrow indicates the popliteal lymph node. The values of the scale bars for fluorescence intensity were adjusted to normalize each image series at comparable values. (B) Quantitative analysis of dye accumulation into the popliteal lymph node. Signal over noise ratio was measured. Values are means ± SE; n = 6 (Bmp9-KO) or n = 9 (WT). *P ≤ .05, significantly different by Mann-Whitney U statistical test.
Discussion
The lymphatic vasculature contains 2 distinct compartments: lymphatic capillaries, important for the uptake of interstitial fluid, and collecting vessels, which transport the lymph toward lymph nodes and back to the circulation. In the present work, we demonstrate that BMP9 is critical for the development of the lymphatic vasculature of these 2 compartments as well as for the formation of lymphatic valves.
The effect of BMP9 on the development of lymphatic capillaries in the skin is in agreement with the previous work published by Niessen et al, who demonstrated that adding the extracellular domain of ALK1, which traps ALK1 ligands at P1, P3, and P5 affected the honeycomb lymphatic vessel pattern of the mouse tail dermis.16 In the present work, we show that BMP9 is the ALK1 ligand involved in vascular lymphatic capillary patterning. Indeed, we found that Bmp9-deficient mice exhibited enlarged lymphatic capillaries in the abdominal skin of neonates and in the ear of adult mice. In addition, our results also provide evidence for an important role of BMP9 on the maturation of large lymphatic-collecting vessels. Indeed, the mesenteric collecting vessels were enlarged, which could result from a hyperplasia because an increase in the number of lymphatic cells was observed. We also demonstrated that these enlarged vessels expressed a higher level of LYVE-1. In vitro, we could show that BMP9 downregulated LYVE-1 expression via ALK1. Although LYVE-1 is found absent in cells constituting valves and downregulated in collecting vessels, the meaning of this phenomenon is unknown and remains to be elucidated.
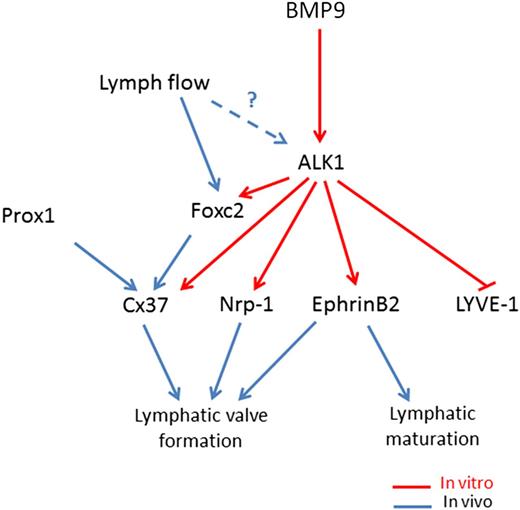
The formation of intraluminal valves accompanies collecting vessel maturation and specification and is crucial to ensure proper unidirectional lymph flow in the lymphatic system. We report here that BMP9 controls this fundamental step. We found that Bmp9-KO embryos and pups presented a significant reduction in the total number of valves at E18.5, P0, and P4 and that this reduction is in a large part from a decrease in the number of mature valves vs immature valves, in particular at P0 and P4. This is in favor of a role for BMP9 both in the initiation of the process and in the maturation of these valves. Still, the few mature valves observed in Bmp9-KO pups did not show any major differences in their overall structure as revealed by Foxc2, integrin α9, and α-SMA staining, suggesting that once the commitment in valve maturation is triggered, these valves correctly mature. We found that BMP9 significantly increased the expression of 2 master genes, Foxc2 and Cx37, involved in valve formation. The reduction in the total number of valves may result from a defect in the early initiation process driven by Foxc2 whereas the defect in valve maturation in Bmp9-KO mice may be the consequence of low Cx37 expression, which is involved in the assembly and delimitation of lymphatic valve territory.7,9 We demonstrated that BMP9 regulates the expression of several important features of the process of lymphatic maturation and valvulogenesis, providing a molecular framework for a stepwise model of lymphatic vessel maturation and valve formation as described in Figure 7. BMP9 acting via ALK1 inhibits LYVE-1 expression, which is concomitant with lymphatic maturation. In parallel, BMP9 also induces the expression of Foxc2, Cx37, ephrinB2, and Nrp-1, which are all involved in valve formation. Interestingly, EphrinB2 has already been demonstrated to be a target of BMP9 in blood endothelial cells.25 Flow has been demonstrated to induce Foxc2 expression and to act in concert with Prox1 to regulate early steps of lymphatic valve morphogenesis.7 ALK1 expression has also been reported to be regulated by flow in zebrafish.26 Therefore, we propose that flow could increase ALK1 expression, which would raise BMP9 signaling to favor the downstream cascade of lymphatic vessel development. Although Prox1 has been established to be the master regulator of LEC fate, its function in lymphatic valves is not completely understood. Accordingly, we observed that BMP9 only induces a slight and transient inhibition in Prox1 mRNA level in LECs. These data suggest that Prox1 may not be the main target involved in BMP9-mediated valve formation.
Working model for BMP9 regulation of lymphatic vessel maturation and valve development. In LECs, BMP9 acting via ALK1 inhibits LYVE-1 expression. In parallel, BMP9 also via Alk1, induces the expression of Foxc2, Cx37, ephrinB2, and Nrp-1, which have all been involved in lymphatic valve formation. Flow has been demonstrated to induce Foxc2 expression and to act in concert with Prox1 to regulate early steps of lymphatic valve morphogenesis.7 ALK1 expression has been reported to be regulated by flow in zebrafish.26 Therefore, we propose that flow could increase ALK1 expression, which would raise BMP9 signaling to favor the downstream cascade of lymphatic vessel development.
Working model for BMP9 regulation of lymphatic vessel maturation and valve development. In LECs, BMP9 acting via ALK1 inhibits LYVE-1 expression. In parallel, BMP9 also via Alk1, induces the expression of Foxc2, Cx37, ephrinB2, and Nrp-1, which have all been involved in lymphatic valve formation. Flow has been demonstrated to induce Foxc2 expression and to act in concert with Prox1 to regulate early steps of lymphatic valve morphogenesis.7 ALK1 expression has been reported to be regulated by flow in zebrafish.26 Therefore, we propose that flow could increase ALK1 expression, which would raise BMP9 signaling to favor the downstream cascade of lymphatic vessel development.
The BMP9/ALK1 signaling pathway has been largely demonstrated to be involved in the regulation of angiogenesis.27,28 It has been established that this pathway is an important regulator of postnatal neovascularization of the mouse retina.15,29 In previous work, we found that Bmp9 deficiency did not alter postnatal blood vascularization, whereas addition of a neutralizing anti-BMP10 antibody in these pups had a dramatic effect on this vascularization, demonstrating that BMP10 could compensate for the loss of BMP9 in retinal angiogenesis.15 In accordance with these data, we did not observe any defects in blood vessels of the ventral skin nor in the ear in Bmp9-KO mice. In contrast, we show here that Bmp9 deficiency is sufficient to affect lymphatic development, and thus that BMP10 cannot compensate BMP9 deficiency for lymphatic development. This does not seem to be the result of the involvement of distinct downstream signaling mechanisms for BMP9 and BMP10, because we found that BMP9-regulated genes in LECS are also targets for BMP10 (data not shown). Another possible explanation may be that active BMP10 might not be accessible to lymphatic vessels. BMPs are required to be locally activated by proteases of the furin/subtilisin proprotein convertase family.30 We could suppose that the activation machinery for BMP10 might not be present within the lymphatic vessel environment.
Importantly, we established here that Bmp9 deficiency has a functional consequence in the draining efficiency of the lymphatic system in both neonates and adult mice. Although we could demonstrate a significant reduction in the draining efficiency, the lymphatic system remained functional. This is consistent with the fact that these mice do not apparently suffer from edema. Therefore, our results would suggest that BMP9 is not fundamental for lymphatic function under normal physiological situations but that the defects observed in these Bmp9-KO mice may have important consequences under conditions in which a more active drainage is necessary. Whether the alteration of BMP9 signaling in mice and in human patients may contribute to lymphedema formation or to metastatic dissemination is now an important question to solve.
In conclusion, our study sheds light on early steps of lymphatic maturation and valve formation, and suggests an important contribution of the growth factor BMP9 in this remodeling process. To our knowledge, this work is the first description of a circulating factor, which could regulates lymphatic valves formation and maturation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Amélie Sabine for sharing her expertise and protocols for mouse tissue dissection and whole mount immunostaining; Dr Jean-Luc Coll and the Plateforme d'imagerie optique du petit animal of INSERM U823 (Institut Albert Bonniot, Grenoble, France) for the access to their macroscope; and Mariela Subileau, Stéphane Hasse, and Pierre Simonet for technical help and the animal care staff of iRTSV for their help in animal husbandry.
This work was supported by INSERM (U1036), Commissariat à l'énergie atomique et aux énergies alternatives (Institut de Recherches en Technologies et Sciences pour le Vivant/laboratoire Biologie du Cancer et de l'Infection et Département des micro-Technologies pour la Biologie et la Santé/Laboratoire d'Electronique de Technologie de l'Information), Université Joseph Fourier, Association pour la Recherche sur le Cancer (postdoctoral grant to G.M. and grant SFI20111203720), the Groupement d’Entreprises Françaises de Lutte contre le Cancer Dauphiné-Savoie, the Comité Départemental de la Loire et de l’Isère de la Ligue contre le cancer, Association Malades du Rendu-Osler, and the Fondation Lefoulon-Delalande (postdoctoral grant to D.C.). Work in S.-J. Lee's laboratory was supported by National Institutes of Health grant HD35887/AR060636.
Authorship
Contribution: S.L., D.C., G.M., and D.V. performed research; C.M. performed genotyping experiments; T.Z. and S.-J.L. generated the Bmp9-KO mice; F.P.N. and I.T. generated DiD-lipidots; J.-J.F., S.B., and D.V. designed research; S.L., D.C., J.-J.F., S.B., and D.V. discussed the results; D.V. and S.B. wrote the manuscript with the help of S.L., D.C., and J.-J.F.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel Vittet, INSERM U1036, 17 rue des Martyrs, CEA Grenoble, 38054 Grenoble cedex 9, France; e-mail: daniel.vittet@cea.fr.
References
Author notes
S.L. and D.C. contributed equally to the study. S.B. and D.V. are co-last authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal