Key Points
Fpr2/3 activation controls platelet/neutrophil aggregates to afford LXA4 synthesis, thus inhibiting vascular inflammation on reperfusion.
Aspirin can jumpstart this circuit by triggering 15-epi-lipoxin synthesis.
Abstract
Endogenous protective pathways mitigate the overshooting of inflammation after sterile or infectious injury. Here we report that formyl peptide receptor 2 (Fpr2/3) null mice display a major phenotype with exacerbated vascular inflammation observed postischemia reperfusion (IR) injury of the mesenteric artery, characterized by marked neutrophil adhesion and extravasation as visualized by intravital microscopy. Analysis of endogenous agonists for Fpr2/3 revealed that lipoxin A4 (LXA4) was generated by platelet/neutrophil aggregates during ischemia: this cellular response was attenuated in Fpr2/3−/− mice; hence, LXA4 levels were lower after 30 minutes’ ischemia, and associated with augmented vascular inflammation in the reperfusion (45-180 minutes) phase. Exogenous delivery of LXA4 attenuated IR-mediated inflammation in Fpr2/3+/+ but not Fpr2/3−/− mice; conversely, an Fpr2/3 antagonist skewed the vascular phenotype of Fpr2/3+/+ mice to that of Fpr2/3−/− animals. Such LXA4-based circuit could be activated by aspirin (30-100 mg/kg), which triggered formation of 15-epi-LXA4 in wild-type mice, yet it was effective in Fpr2/3−/− mice. In summary, we propose that during ischemia, neutrophil Fpr2/3 controls platelet/neutrophil aggregates with the rapid generation of circulating LXA4, which in turn modulates downstream vascular inflammatory responses evident during the reperfusion phase.
Introduction
There is recent appreciation that the host response to infections and insults is tightly regulated with the engagement of a series of lipid mediators, cytokines, and adhesion molecules to promote extravasation, immune cell trafficking, and removal of pathogen.1,2 These events are followed by release of other mediators that quench these processes, stopping cell trafficking, promoting apoptosis and efferocytosis of extravasated cells, and leading to tissue repair.3 Effectors of resolution ensure that this response remains localized within the inflamed tissue site and it is extinguished within an adequate timeline. The glucocorticoid cortisol exerts a central role in resolution or, in more general terms, on the host defense response in stress conditions.4 In a homeostatic fashion, there is appreciation that “…the beginning programs the end,” and therefore the pro-inflammatory phase induces resolution, to assure a strict, temporally and spatially regulated response.5
Within the context of resolution, a prominent role is played by rapidly produced lipid mediators, such as 5S,6R,15S-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid (lipoxin A4 [LXA4)]) or resolvins6 as well as proteins including erythropoietin and Annexin A1 (AnxA1). In particular, LXA4 and AnxA1 are prototypes of pro-resolving mediators in view of their different nature (lipid vs protein), mode of activation (transcellular biosynthesis vs cytoplasmic pools mobilization), and engagement by widely used therapeutics (aspirin [ASA] for LXA4 or glucocorticoids for AnxA1). However, they are also interconnected by sharing the G protein–coupled receptor FPR2/ALX (formyl-peptide receptor type 2 or LXA4 receptor; termed Fpr2/3 in the mouse).7,8
Although inflammation is a life-saving process and must be highly efficient against bacteria invasion, “inflammation from within”—that is, in the vasculature—must be tightly controlled to avoid widespread damage. Thus, low doses of LXA4 inhibit polymorphonuclear cells (PMN) accumulation both in postischemic mesenteric tissues9,10 and in distant organs such as the lung11 ; similarly, AnxA1 and erythropoietin are potent inhibitors of myocardial infarct, stroke, and vascular reperfusion injury.12-14 In situations where reperfusion occurs, such as renal transplantation, LXA415 and resolvins16 are highly protective, and the same has been reported recently for AnxA1 mimetics.17
Here we compared vascular inflammation indexes in wild-type and Fpr2/3−/− mice,18 uncovering a novel vasculo-protective pathway centered on LXA4. During the ischemic phase, platelet/neutrophil aggregates are formed, affording transcellular biosynthesis of this bioactive lipid. This process is also activated by low-dose ASA administration.
Material and methods
Animals
Male Fpr2/3+/+ or Fpr2/3−/−18 littermate mice (3-4 weeks old, ∼20 g body weight) were maintained on a standard chow pellet diet and had free access to water, with a 12-hour light-dark cycle. Animals were used according to the guidelines laid down by the Ethical Committee for the Use of Animals, Barts and The London School of Medicine on the basis of “Animal Research: Reporting In Vivo Experiments” protocol. Animal work was performed according to Home Office Regulations (Guidance on the Operation of Animals, Scientific Procedures Act, 1986).
Intravital microscopy after ischemia-reperfusion in mouse mesenteric microcirculation
Intravital microscopy was performed as previously reported.10,19 Mice were anesthetized and placed in the supine position on a heating pad (37°C). Mesenteric ischemia was induced with a microaneurysm clip (Harvard Apparatus, Kent, UK), clamping the superior mesenteric artery for 30 minutes. The clip was then removed, and reperfusion was allowed for between 45 and 180 minutes. Sham-operated animals underwent the same surgical procedure except for the clamping of the superior mesenteric artery.
The vascular bed was exposed and positioned under the microscope; recording started after a 5-minute equilibration period, followed by offline analyses, as reported.10,19 These were made in 1 to 3 randomly selected postcapillary venules (diameter, 20-40 µm; visible length ≥100 μm) for each mouse. Thus, rolling leukocyte flux was obtained by the number of leukocytes passing a reference point in the venule per minute (cells/minute); leukocyte adhesion reflected cells stationary for 30 seconds or longer; leukocyte emigration was calculated by the number of cells in a 100 × 50 μm2 area on both sides of the 100-μm vessel segment. For all vessels, red blood cell centerline velocity was measured with an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, Dallas, TX) and venular wall shear rate was determined based on the Newtonian definition: wall shear rate = 8000 [(red blood cell velocity/1.6)/venular diameter].
Confocal imaging protocol
Immunofluorescence staining of whole mesentery was carried out on tissues fixed in paraformaldehyde 4% for 15 minutes blocked for 1 hour in phosphate-buffered saline containing 1% Triton X-100, 1% bovine serum albumin, and 3% natural goat serum, and washed 3 times. Incubation with specific antibodies for vascular endothelium (clone BV14, eBioscience, Hatfield, UK), neutrophils (clone MRP14-647, in-house labeled), and/or monocytes/macrophages (CD115, AFS98, Biolegend, London, UK) was followed by extensive washes in phosphate-buffered saline and mounted on polylysine-coated slides (Invitrogen, Paisley, UK). Confocal images were analyzed using a Zeiss LSM5 PASCAL confocal laser-scanning microscope (Zeiss, Welwyn Garden City, UK).
Assessment of platelet/neutrophil aggregates in mouse blood
Whole blood flow cytometry was performed to quantify the extent of neutrophil/platelet complexes in blood taken from mice after ischemia-reperfusion (IR), ischemia alone, or sham procedure. Blood aliquots (collected in Hirudin prefilled blood tubes, Dynabyte GmbH, Munich, Germany) were stained with Ly6G-APC (clone RB6-8C5, 1 μg/mL; eBioscience) to identify neutrophils and CD41-PE (MWReg30, 1 μg/mL; eBioscience) to identify platelets. Red blood cells were then lysed, and cell pellet was washed and fixed before sample analysis with a FACScalibur flow cytometer (BD Bioscience, Oxford, UK) acquiring ≥10 000 events. Scatter plot analysis identified the neutrophil population (>95% Ly6G+), and the extent of CD41-positive events therein was then quantified. Results are reported as percentage of Ly6G+/CD41+ (double positive) events.
Chimeric experiment: platelet labeling and reinfusion
Blood from a donor mouse was withdrawn in syringes prefilled with 85 mM sodium citrate, 62.2 mM citric acid, and 110 mM glucose (ACD buffer; Sigma-Aldrich, St. Louis, MO). Platelet-rich plasma was obtained by centrifugation (118 × g, 8 minutes) before platelet isolation by centrifugation at 735 g for 10 minutes. Platelets were gently resuspended, counted, and labeled by using carboxyfluorescein succinimidyl ester (CFSE, 90 µM; 10 minutes). After confirming absence of aggregates by light microscopy, 100 × 106 platelets in 120 µL were injected through the jugular vein of a recipient mouse over 5-minute infusion. The animal was then rested for 5 minutes to allow platelet circulation before starting experiment (ischemia or sham procedure) as described previously.
Pharmacological treatments
Different compounds were administered as tools to define specific biochemical pathways. N-tert-butoxycarbonyl-L-Phe-D-Leu-L-Phe-D-Leu-L-Phe (Boc2; MPBiomedicals, Cambridge, UK) was given intravenously via the jugular vein before ischemia or immediately before reperfusion, at 10 µg per mouse.10 LXA4 was injected before ischemia at doses of 1-100 ng per mouse, as informed from previous experiments.10,13 In a separate set of experiments, mice were treated with anti-inflammatory doses of ASA (30-300 mg/kg intraperitoneally; Sigma-Aldrich) or indomethacin (0.3-3 mg/kg intraperitoneally, Sigma-Aldrich)20 given before the IR procedure.
Determination of plasma mediator levels
Enzyme-linked immunosorbent assays.
LXA4 and 5S,6R,15R-trihydroxyl-7,9,13-trans-11-cis-eicosatetraenoic acid (15-epi-LXA4) were assayed using ELISA kit purchased from Neogen Corporation. Lipid extraction and determination were carried out according manufacturer’s specifications. Thromboxane A2 and prostacyclin formation were quantified with enzyme immunoassay measuring their stable analogs Thromboxane B2 (R&D Systems) and 6-keto-prostaglandin F1α (Cayman Chemicals). Mouse serum amyloid protein A (SAA) levels were determined by using specific kit from Immunology Consultants Laboratory Inc.
Targeted LC-MS/MS-based lipidomics of ischemic plasma samples.
To simultaneously separate lipids of interest and 3 deuterated internal standards (LXA4-d5, leukotriene B4 (LTB4)-d4, hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (5-HETE-d8); Cayman Chemicals), liquid chromatography (LC)-mass spectrometry (MS)/MS analysis was performed with an Agilent LC1290 Infinity coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Les Ulis, France) equipped with electro-spray ionization operating in negative mode. The column (2.1 mm × 50 mm × 1.8 μm; ZorBAX SB-C18 column, Agilent Technologies) was eluted at a flow rate of 0.35 mL/min with acetonitrile/formic acid/water (25/0.1/75; v/v/v) gradually ramped to 100/0.1/0 (v/v/v) over 10 minutes. Instrument control and data acquisition were performed using MassHunter software (Agilent Technologies). Ion pair transitions from previously reported single-reaction monitoring methods were used for profiling and quantitation of LXA4 (351/115), Lipoxin B4 (351/129), 5-HETE (319/115), 12-HETE (319/208), 15-HETE (319/219), and LTB4 (335/195). Criteria for identification were: LC retention time of the diagnostic ion on the MS/MS spectrum matching those of synthetic standards. A linear regression with a weight factor of 1/X was applied for each compound. By the use of this factor, a calibration line with a coefficient of correlation >0.998 and an accuracy ranging from 85% to 115% was obtained. The limit of detection and the limit of quantification were determined for the 13 compounds using signal-to-noise ratio. All values under the limit of quantification were not considered. Blank samples were also evaluated, and their injection showed no interference (no peak detected) during the analysis.
Statistics
All results are reported as mean ± SEM of n distinct observations. Differences between 2 groups were determined with the Student t test; more than 2 groups were analyzed by analysis of variance upon satisfaction of homogeneity of variances. Intergroup analyses were assessed with the Dunnett or Bonferroni tests, as appropriate. In all cases, P < .05 was taken as significant to reject the null hypothesis.
Results
Intravital microscopy analysis of IR injury in the Fpr2/3−/− mesenteric microcirculation
Clamping of the superior mesenteric artery for 30 minutes, followed by reperfusion, provoked a time-dependent vascular inflammatory response. As expected, rapid induction of cell flux occurred at 45 minutes after reperfusion, with no statistical difference between Fpr2/3+/+ and Fpr2/3−/− mice (not shown). This was followed by a robust cell adhesion response that was significantly exacerbated in Fpr2/3−/− mice. Cell adhesion to vascular endothelium peaked at 45 minutes and 90 minutes in Fpr2/3+/+ and Fpr2/3−/− mice, respectively (Figure 1A), with cell emigration augmenting in a delayed fashion. A maximal response of ∼10 emigrated cells in Fpr2/3+/+ mice was reached at 90 minutes after reperfusion, whereas this response progressed further up to the 180-minute time-point in Fpr2/3−/− animals (∼20 leukocytes; Figure 1B).
Augmented vascular inflammation in Fpr2/3−/−mice as assessed by intravital microscopy. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to 30 minutes’ clamping of the superior mesenteric artery, followed by a reperfusion phase lasting 45 to 180 minutes. Postcapillary venules were imaged and recorded for offline quantitation of white blood cell interaction with the endothelium. In parallel experiments, tissue was excised and analyzed by confocal microscopy. (A) Number of cell adhesion and (B) emigration in the postcapillary venules, as measured at different times after reperfusion. Representative light microscopy images are shown for sham (C-D) and 90-minute post-reperfusion (E-F) vessels. (G-H) Confocal images for postcapillary venules from Fpr2/3+/+ and Fpr2/3−/− mice, respectively, after 90 minutes of IR protocol. Green, vessel vascular endothelium staining; blue, neutrophil MRP14 marker. Data are mean ± SEM of 6 to 8 mice per group. *P < .05, **P < .01 vs respective Fpr2/3+/+ value.
Augmented vascular inflammation in Fpr2/3−/−mice as assessed by intravital microscopy. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to 30 minutes’ clamping of the superior mesenteric artery, followed by a reperfusion phase lasting 45 to 180 minutes. Postcapillary venules were imaged and recorded for offline quantitation of white blood cell interaction with the endothelium. In parallel experiments, tissue was excised and analyzed by confocal microscopy. (A) Number of cell adhesion and (B) emigration in the postcapillary venules, as measured at different times after reperfusion. Representative light microscopy images are shown for sham (C-D) and 90-minute post-reperfusion (E-F) vessels. (G-H) Confocal images for postcapillary venules from Fpr2/3+/+ and Fpr2/3−/− mice, respectively, after 90 minutes of IR protocol. Green, vessel vascular endothelium staining; blue, neutrophil MRP14 marker. Data are mean ± SEM of 6 to 8 mice per group. *P < .05, **P < .01 vs respective Fpr2/3+/+ value.
Representative images of postcapillary venules are shown in Figure 1C-H. Noninflamed vessels in sham mice (panels C and D) and marked accumulation of leukocytes in mice subjected to IR at 90 minutes after reperfusion, with higher counts observed in Fpr2/3−/− mice (P < .01) (supplemental Figure 1: images at 45-180 minutes after reperfusion). These leukocytes were all neutrophils, as demonstrated by specific staining for MRP14 using confocal microscopy (Figure 1D). CD115 staining was also performed to identify activated monocytes, but this cell type was not detected at any of the time points analyzed (data not shown). Supplemental Table 1 summarizes the hemodynamic parameters measured in these microvascular beds, indicating no major changes between genotypes.
Effect of Fpr2/3 agonists and antagonists on vascular inflammation post-IR
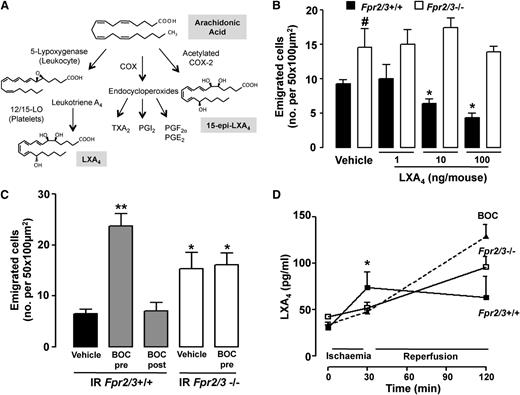
LXA4 is a pro-resolutive end product of a transcellular-based enzymatic machinery6 (see scheme in Figure 2A). Treatment of Fpr2/3+/+ mice with LXA4 provoked a marked attenuation of the inflammatory response in the mesenteric vasculature, as assessed 90 minutes after reperfusion and reported in Figure 2B as the number of emigrated cells (similar results for cell adhesion; not shown). A dose of 10 ng (∼30 pmol) per mouse of LXA4 afforded ∼50% reduction in cell emigration in Fpr2/3+/+ mice, but was inactive in Fpr2/3−/− animals (Figure 2B). Treatment of Fpr2/3+/+ mice with the Fpr2/3 antagonist Boc2 before, but not after, ischemia unveiled a phenotype in Fpr2/3+/+ mice similar to that of Fpr2/3−/− mice, that is an increase in the extent of neutrophil adhesion (not shown) and emigration (Figure 2C). Boc2 did not modify the cellular response in Fpr2/3−/− mice (Figure 2C). These results indicate that Fpr2/3 controls vascular reactivity to ensure adequate counterregulation of the inflammatory response during IR of the mesenteric microcirculation.
Endogenous Fpr2/3 modulates vascular inflammation. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to 30 minutes’ clamping of the superior mesenteric artery, followed by a 90-minute reperfusion phase. Postcapillary venules were imaged and recorded for offline quantitation of white blood cell interaction with the endothelium. (A) Arachidonic acid metabolism into LXA4 by the concerted action of 5-lipoxygenase (LOX) and 12/15-LOX. Acetylation of cyclo-oxygenase 2 (COX) by ASA leads to 15-epi-LXA4 biosynthesis. Prostanoids produced by COX 1 or 2 isoforms are also indicated, and they include thromboxane (TX) A2 and prostaglandin (PG) of series I, E, and F. (B) Mice were given LXA4 (1, 10, or 100 ng/mouse intravenously) or vehicle (100 µL). Mean ± SEM of 6 mice; *P < .05 vs vehicle; #P < .05 vs Fpr2/3+/+. (C) Administration of Boc2 (10 µg intravenously) before (pre) or after (post) ischemia (30 minutes) affects the extent of cell emigration as measured 90 minutes after reperfusion in Fpr2/3+/+ but not Fpr2/3−/− mice. Mean ± SEM of 6 to 8 mice; *P < .05, **P < .01 vs Fpr2/3+/+ vehicle. (D) Plasma aliquots obtained at the reported phases of the IR protocol were analyzed for LXA4 content by enzyme immunoassay. Mean ± SEM of 8 mice; *P < .05 vs Fpr2/3−/− or Boc2-treated Fpr2/3+/+ mice.
Endogenous Fpr2/3 modulates vascular inflammation. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to 30 minutes’ clamping of the superior mesenteric artery, followed by a 90-minute reperfusion phase. Postcapillary venules were imaged and recorded for offline quantitation of white blood cell interaction with the endothelium. (A) Arachidonic acid metabolism into LXA4 by the concerted action of 5-lipoxygenase (LOX) and 12/15-LOX. Acetylation of cyclo-oxygenase 2 (COX) by ASA leads to 15-epi-LXA4 biosynthesis. Prostanoids produced by COX 1 or 2 isoforms are also indicated, and they include thromboxane (TX) A2 and prostaglandin (PG) of series I, E, and F. (B) Mice were given LXA4 (1, 10, or 100 ng/mouse intravenously) or vehicle (100 µL). Mean ± SEM of 6 mice; *P < .05 vs vehicle; #P < .05 vs Fpr2/3+/+. (C) Administration of Boc2 (10 µg intravenously) before (pre) or after (post) ischemia (30 minutes) affects the extent of cell emigration as measured 90 minutes after reperfusion in Fpr2/3+/+ but not Fpr2/3−/− mice. Mean ± SEM of 6 to 8 mice; *P < .05, **P < .01 vs Fpr2/3+/+ vehicle. (D) Plasma aliquots obtained at the reported phases of the IR protocol were analyzed for LXA4 content by enzyme immunoassay. Mean ± SEM of 8 mice; *P < .05 vs Fpr2/3−/− or Boc2-treated Fpr2/3+/+ mice.
Next, we monitored endogenous generation of Fpr2/3 agonists including the anti-inflammatory and pro-resolving lipid mediator LXA4 and the pro-inflammatory agonist SAA. Enzyme-linked immunoassay analysis indicated significant changes in plasma LXA4 levels (∼twofold increase) in Fpr2/3+/+ mice after 30 minutes of ischemia (Figure 2D). This early modulation did not occur in either Fpr2/3−/− or Fpr2/3+/+ mice treated with Boc2, in which LXA4 plasma levels were elevated solely after 90 minutes of reperfusion (Figure 2D). In comparison, levels of SAA levels did not change during the course of the IR procedure in either of the mouse genotypes (supplemental Figure 2). LC/MS/MS-based lipidomic analysis was performed to afford multiple lipid determination. Changes in LXA4 plasma levels were unequivocally determined by chromatography peak identification and diagnostic fragments (Figure 3A). Quantification of these results demonstrated a substantial increase in LXA4 at after 30 minutes’ ischemia in Fpr2/3+/+, but not Fpr2/3−/−, mice (Figure 3B). Interestingly, lipoxin B4 (LXB4), 5-HETE, 12-HETE, and LTB4 plasma levels did not change upon ischemia (Figure 3B). In contrast, 15-HETE was significantly elevated, but this increment occurred in both genotypes (Figure 3B).
LC/MS/MS-based lipidomic analysis. (A) Left panel. Spectrum of relative peaks for LXA4 and LXB4. Right panel, LXA4 signature fragment ions reported as m/z, highlighting the 2 diagnostic fragments at 115 and 351 (351 is the ion mass). (B) Measurements of LXA4 levels and other bioactive lipid mediators in mouse plasma. Besides LXA4, values for LXB4, leukotriene (LT) B4, 5-HETE, 12-HETE, and 15-HETE are reported as measured in wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice, in sham or ischemic (30 minutes’ occlusion of the mesenteric artery) conditions. Mean ± SEM of 6 mice; *P < .05 vs respective sham-operated mice.
LC/MS/MS-based lipidomic analysis. (A) Left panel. Spectrum of relative peaks for LXA4 and LXB4. Right panel, LXA4 signature fragment ions reported as m/z, highlighting the 2 diagnostic fragments at 115 and 351 (351 is the ion mass). (B) Measurements of LXA4 levels and other bioactive lipid mediators in mouse plasma. Besides LXA4, values for LXB4, leukotriene (LT) B4, 5-HETE, 12-HETE, and 15-HETE are reported as measured in wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice, in sham or ischemic (30 minutes’ occlusion of the mesenteric artery) conditions. Mean ± SEM of 6 mice; *P < .05 vs respective sham-operated mice.
A closer assessment of these findings revealed a significant negative correlation between postischemic plasma LXA4 concentrations and the extent of emigrated cells 90 minutes after reperfusion (supplemental Figure 3). Delivery of Boc2 to block endogenous Fpr2/3 disrupts the process(es) leading to LXA4 biosynthesis, abrogating this correlation (supplemental Figure 3).
Evidence of platelet/neutrophil aggregate formation during ischemia
A major biosynthetic pathway for LXA4 within the vasculature occurs by the concerted action of platelet 12/15 lipoxygenase and neutrophil 5-lipoxygenase21 ; hence, we questioned whether the differences in LXA4 biosynthesis could be due to differences in the number of platelet/neutrophil aggregates forming during ischemia.
We used a flow cytometry–based approach to study the granulocyte population in whole blood (see scattering dot plot in supplemental Figure 4). Using a specific antibody against Ly6G or CD41 to identify PMN or platelets, respectively, aggregate formation was monitored in ischemia. Whereas no significant differences were measured between Fpr2/3+/+ and Fpr2/3−/− in blood taken in sham conditions (∼25–30% of neutrophils attached to platelets), a significant increase in aggregates was quantified postischemia in Fpr2/3+/+ mice (∼70% of neutrophils with platelets) (Figure 4A). The percentage of aggregates quantified in Fpr2/3−/− mice after 30 minutes’ ischemia was significantly lower (∼50%; Figure 4B).
Formation of platelet/neutrophil complexes after ischemia. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to clamping of the superior mesenteric artery, with blood being rapidly withdrawn at the end of the 30-minute ischemic period. Sham animals were anesthetized but not subjected to ischemia. (A) Flow cytometry scattergrams illustrating neutrophils (APC-Ly6G+)- and platelet (PE-CD41+)-positive events. The 4 quadrants show CD41+ events in the Ly6G+-gated population. Numbers in upper right quadrants indicate the percent of double-positive events (ie, the proportion of neutrophil/platelet complexes). (B) Cumulative data for platelet/neutrophil complexes. Mean ± SEM of 6 mice; **P < .01 vs respective sham group. (C-D) Chimeric experiment performed injecting CFSE-labeled platelets in recipient animals with the opposite genotype. Labeled platelets were injected immediately before ischemia, and blood was collected at the end of the 30-minute period; flow cytometry for APC-Ly6G+ and CFSE+ was then run, essentially as in (A). (C) Representative dot plots with the distribution of the 2 fluorochromes on all acquired events; (D) cumulative quantitative data. Mean ± SEM of 4 mice per group. *P < .05 and **P < .01 vs respective sham-operated mice.
Formation of platelet/neutrophil complexes after ischemia. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to clamping of the superior mesenteric artery, with blood being rapidly withdrawn at the end of the 30-minute ischemic period. Sham animals were anesthetized but not subjected to ischemia. (A) Flow cytometry scattergrams illustrating neutrophils (APC-Ly6G+)- and platelet (PE-CD41+)-positive events. The 4 quadrants show CD41+ events in the Ly6G+-gated population. Numbers in upper right quadrants indicate the percent of double-positive events (ie, the proportion of neutrophil/platelet complexes). (B) Cumulative data for platelet/neutrophil complexes. Mean ± SEM of 6 mice; **P < .01 vs respective sham group. (C-D) Chimeric experiment performed injecting CFSE-labeled platelets in recipient animals with the opposite genotype. Labeled platelets were injected immediately before ischemia, and blood was collected at the end of the 30-minute period; flow cytometry for APC-Ly6G+ and CFSE+ was then run, essentially as in (A). (C) Representative dot plots with the distribution of the 2 fluorochromes on all acquired events; (D) cumulative quantitative data. Mean ± SEM of 4 mice per group. *P < .05 and **P < .01 vs respective sham-operated mice.
To determine whether platelet or neutrophil Fpr2/3 was more important in evoking this vascular response during ischemia, a series of chimeric experiments was performed. Platelets from Fpr2/3+/+ or Fpr2/3−/− mice were labeled with CFSE and injected into Fpr2/3−/− or Fpr2/3+/+ mice, respectively, before occlusion of the mesenteric artery. Flow cytometry analysis at 30 minutes’ ischemia revealed a functional role for neutrophil Fpr2/3 because a significant response over sham was obtained with Fpr2/3−/− platelet infusion into Fpr2/3+/+ mice, whereas no increase in aggregate number was quantified when wild-type platelets were infused into Fpr2/3−/− mice (Figure 4C-D). Furthermore, control experiments infusing Fpr2/3+/+ platelets into Fpr2/3+/+ recipient mice were conducted, with approximate values of 40% Ly6G/CFSE double positive events; similarly, Fpr2/3−/− platelets into Fpr2/3−/− recipient mice yielded ∼20% Ly6G/CFSE double-positive counts (data not shown).
Low-dose ASA, but not indomethacin, requires functional Fpr2/3
The data reported so far indicate that LXA4 can modulate vascular inflammation post-IR in Fpr2/3+/+ mice but not in Fpr2/3−/− animals. Next, we determined if vasculoprotective drugs could use this novel circuit. Here we tested the actions of ASA, which has been shown to trigger the biosynthesis of a LXA4 epimer,22 and indomethacin, which does not.
Treatment of mice with ASA (30-300 mg/kg) produced a dose-dependent inhibition of vascular inflammation as measured 90 minutes after reperfusion (Figure 5). Although the top dose of 300 mg/kg was equally active in Fpr2/3−/− mice, lower doses of 30 and 100 mg/kg ASA were no longer able to inhibit cell adhesion (Figure 5A) or emigration (Figure 5B) in Fpr2/3−/− mice. Indomethacin markedly inhibited white blood cell interaction with post-capillary venules in both genotypes, as quantified by intravital microscopy (supplemental Figure 5).
ASA is less effective in Fpr2/3-deficient mice. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to 30 minutes’ clamping of the superior mesenteric artery, followed by a 90-minute reperfusion phase. Mice received vehicle (1 mL/kg) or the reported doses of ASA intraperitoneally 30 minutes before ischemia. Bars report the number of neutrophils adherent (A) or emigrated (B) into the subendothelial tissue. Mean ± SEM of 8 mice per group. *P < .05, **P < .01, ***P < .001 vs the respective vehicle group.
ASA is less effective in Fpr2/3-deficient mice. Wild-type (Fpr2/3+/+) and null (Fpr2/3−/−) mice were subjected to 30 minutes’ clamping of the superior mesenteric artery, followed by a 90-minute reperfusion phase. Mice received vehicle (1 mL/kg) or the reported doses of ASA intraperitoneally 30 minutes before ischemia. Bars report the number of neutrophils adherent (A) or emigrated (B) into the subendothelial tissue. Mean ± SEM of 8 mice per group. *P < .05, **P < .01, ***P < .001 vs the respective vehicle group.
By acetylating the catalytic site of cyclo-oxygenase, ASA blocks the conversion of arachidonic acid into prostaglandins but leads instead to the generation of epi-LXA4 which triggers pro-resolving responses through engagement of Fpr2/3.7 To ascertain whether this mechanism operated in our model, we measured plasma lipid mediators in mice treated with ASA (Table 1). IR augmented levels of thromboxane A2 (measured as its stable metabolite TXB2) and prostacyclin compared with sham mice; ASA (100 mg/kg) attenuated this response. The effect was also present in Fpr2/3−/− mice, indicating that it is independent from platelet/neutrophil aggregates and, rather, functionally related to cyclo-oxygenase blockade. We could not detect 15-epi-LXA4 in vehicle-treated mice, with or without IR, but this LXA4 epimer was measured in both genotypes upon treatment with ASA (Table 1). Therefore, at appropriate doses, ASA induces biosynthesis of an Fpr2/3 agonist, which attenuates vascular inflammation in Fpr2/3+/+ but not Fpr2/3−/− mice.
ASA-induced eicosanoid generation in mouse plasma
| Genotype . | Treatment . | TXB2 . | 6-keto-PGF1α . | 15-epi-LXA4 . |
|---|---|---|---|---|
| (ng/mL) . | ||||
| Fpr2/3+/+ | Sham | 0.91 ± 0.09 | 0.14 ± 0.09 | ND |
| IR | 3.45 ± 0.18* | 3.28 ± 0.65* | ND | |
| IR + ASA | 2.07 ± 0.03*† | 0.87 ± 0.44† | 483 ± 252 | |
| Fpr2/3−/− | Sham | 1.09 ± 0.26 | 0.59 ± 0.17 | ND |
| IR | 2.67 ± 0.88* | 2.01 ± 0.64* | ND | |
| IR + ASA | 2.15 ± 0.03*† | 0.16 ± 0.08† | 789 ± 61 | |
| Genotype . | Treatment . | TXB2 . | 6-keto-PGF1α . | 15-epi-LXA4 . |
|---|---|---|---|---|
| (ng/mL) . | ||||
| Fpr2/3+/+ | Sham | 0.91 ± 0.09 | 0.14 ± 0.09 | ND |
| IR | 3.45 ± 0.18* | 3.28 ± 0.65* | ND | |
| IR + ASA | 2.07 ± 0.03*† | 0.87 ± 0.44† | 483 ± 252 | |
| Fpr2/3−/− | Sham | 1.09 ± 0.26 | 0.59 ± 0.17 | ND |
| IR | 2.67 ± 0.88* | 2.01 ± 0.64* | ND | |
| IR + ASA | 2.15 ± 0.03*† | 0.16 ± 0.08† | 789 ± 61 | |
Blood was collected from sham mice or animals subjected to 30-min clamping of the superior mesenteric artery followed by 90-min IR. Some mice received ASA (100 mg/kg orally 1 h before beginning of ischemia). Plasma was prepared and analyzed for eicosanoids content using specific enzyme immunoassays (see “Methods”). Data are mean ± SEM of 4 samples per group.
ND, not detectable.
P < .05 vs sham.
P < .05 vs respective IR value.
Discussion
Appreciation of the homeostatic functions of pro-resolving and tissue protective mediators and pathways is emerging in the context of immune responses ranging from colitis to joint disease, from ocular to periodontal pathologies.3 However, relevance of these effectors of resolution in vascular damage is scantily investigated.
This study originated from the observation that Fpr2/3−/− mice experience more aggressive neutrophil trafficking in postcapillary venules in mesenteric IR. This led us to unveil an endogenous protective signal, mediated by neutrophil Fpr2/3, controlling aggregate formation with platelets in the bloodstream. The end point consists in the generation of LXA4 that would then limit the degree of vascular inflammation. In absence of Fpr2/3, the fine-tuning exerted by this novel circuit is lost, leading to excessive vascular inflammation and potential tissue injury. Figure 6 schematizes these novel findings.
Fpr2/3 governs LXA4biosynthesis and downstream inflammatory responses in the microcirculation during ischemia and reflow. Ischemic episodes led to an Fpr2/3-mediated formation of platelet/PMN aggregates, with a signal starting from the receptor expressed on the leukocyte. Aggregates afford the transcellular biosynthesis of LXA4 via the concerted action of 5-lipoxygenase (5-LOX) and 12/15-LOX, in PMN and platelet, respectively. High levels of LXA4 assured an appropriate (physiological) vascular inflammation during reperfusion (that subsides within 3-4 hours; not shown). Inappropriate aggregate formation during ischemia leads to low LXA4 levels, with consequent higher degree of leukocyte adhesion and extravasation during reperfusion (reflow). The effector functions of LXA4 on vascular inflammation processes are also mediated by Fpr2/3, because they are absent in mice deficient for this receptor. Among several mechanisms, acetylation of cyclo-oxygenase-2 (COX2) by ASA jumpstarts this protective circuit, with formation of 15-epi-LXA4, activating Fpr2/3 and moderating vascular reactivity. In summary, mouse Fpr2/3 and, we propose its human counterpart ALX/FPR2, governs the extent of vascular inflammation in conditions of occlusion and reflow, by promoting formation of LXA4, and also mediating the direct effects of this bioactive lipid mediator on leukocyte adhesion and emigration.
Fpr2/3 governs LXA4biosynthesis and downstream inflammatory responses in the microcirculation during ischemia and reflow. Ischemic episodes led to an Fpr2/3-mediated formation of platelet/PMN aggregates, with a signal starting from the receptor expressed on the leukocyte. Aggregates afford the transcellular biosynthesis of LXA4 via the concerted action of 5-lipoxygenase (5-LOX) and 12/15-LOX, in PMN and platelet, respectively. High levels of LXA4 assured an appropriate (physiological) vascular inflammation during reperfusion (that subsides within 3-4 hours; not shown). Inappropriate aggregate formation during ischemia leads to low LXA4 levels, with consequent higher degree of leukocyte adhesion and extravasation during reperfusion (reflow). The effector functions of LXA4 on vascular inflammation processes are also mediated by Fpr2/3, because they are absent in mice deficient for this receptor. Among several mechanisms, acetylation of cyclo-oxygenase-2 (COX2) by ASA jumpstarts this protective circuit, with formation of 15-epi-LXA4, activating Fpr2/3 and moderating vascular reactivity. In summary, mouse Fpr2/3 and, we propose its human counterpart ALX/FPR2, governs the extent of vascular inflammation in conditions of occlusion and reflow, by promoting formation of LXA4, and also mediating the direct effects of this bioactive lipid mediator on leukocyte adhesion and emigration.
LXA4 was identified by Serhan and colleagues in the late 1980s,23 whereas ASA-triggered lipid mediators were discovered later22,24 ; these intriguing autacoids are formed by transcellular biosynthesis in which a first enzyme/cell generates an intermediate product used as substrate by a second enzyme/cell. In the mouse, 1 such pathway leading to LXA4 biosynthesis is through the concerted action of neutrophils and platelets, providing the 5-lipoxygenase and the 12/15-lipoxygenase (2 distinct enzymes in the human system), respectively21 (see Figure 6 for scheme). We demonstrate here that during mesenteric IR, this biosynthetic pathway is activated. In particular, platelet/neutrophil aggregation would “provide the machinery” for generation of LXA4 during ischemia; it is plausible that during reperfusion, the endothelial cell/neutrophil pair (recently reviewed by Eltzschig and Eckle25 ) will support and expand the biosynthesis of these lipids. In our experimental settings, the leukocyte involved is the neutrophil and we could not detect trafficking of inflammatory monocytes (or any other bloodborne cell) within the applied timeframe.
The present project began by assessing if Fpr2/3 had any functional relevance in IR. In sterile inflammation, this receptor is the main transducer of the pro-resolving actions of LXA4 and AnxA1. Pharmacological delivery of either mediator engages Fpr2/3 to activate circuits of resolution, including attenuation of leukocyte trafficking,18 promotion of apoptosis,26 and efferocytosis,27,28 favoring egress of leukocyte from the tissue.29 Attenuation of postischemic leukocyte recruitment is crucial to prevent vascular and parenchymal damage as shown in many experimental settings in animals and also in human intestine, where removal of neutrophils for perfusates yielded consistent diminution of tissue injury.30 Pro-resolving processes have been detailed, mostly, in experimental models of tissue inflammation, including peritonitis, pleurisy, and dorsal air pouches, with important confirmation in experimental and human colitis.31,32 More recent studies have corroborated these pharmacological findings with investigations in transgenic mice, including AnxA1−/−,33 ,34 and 12/15-lipoxygenase−/−35 animals, where exacerbated leukocyte trafficking and delayed resolution have been noted.
The pivotal functions of endogenous anti-inflammatory pro-resolving mediators in vascular inflammation have been reported (see the “Introduction”). Searching for endogenous Fpr2/3 agonists, we focused our attention on LXA4, AnxA1, and SAA (reviewed previously by Chiang et al7 ). We measured consistent increases in LXA4 levels during ischemia, with marked amounts seen after reperfusion. SAA levels did not change during the time course of our experiments and remained relatively constant to sham concentration in the 2 genotypes. AnxA1 could not be measured in the plasma, because in the presence of a high calcium concentration, it associates with cell plasma membranes.36 Interestingly, following the clamping and reopening of the superior mesenteric artery in AnxA1−/− mice, we did not detect differences in platelet/neutrophil aggregate formation (at the end of ischemia) or leukocyte/endothelium interaction (up to 180 minutes after reperfusion; data not shown).
The positive association between postischemia LXA4 and the extent of vascular reactivity after reperfusion emerged on several levels. First, there was a highly significant negative correlation between plasma concentration of this bioactive lipid during ischemia and extent of neutrophil adhesion and emigration; second, when a comparison between Fpr2/3+/+ and Fpr2/3−/− was conducted, lack of LXA4 biosynthesis in the null mouse led to marked inflammation. Finally, administration of Boc2 preischemia abrogated the production of endogenous LXA4 with consequent downstream imbalance in vascular reactivity.
The augmented LXA4 levels measured postischemia are likely, though not solely, the result of formation of platelet/neutrophil aggregates and cell-to-cell crosstalk necessary to bring in close proximity the biosynthetic machinery required for its generation.37 The data obtained with the chimeric experiments suggest that this vasculoprotective circuit centered on LXA4 is activated through a neutrophil event and requires Fpr2/3 (Figure 6), whereas we could not obtain evidence for an involvement of SAA or AnxA1. We therefore propose a model in which ischemia initially leads to some sort of neutrophil activation, favoring the formation of aggregates with platelets; this phenomenon would provide biosynthesis of LXA4, which would then act on its receptor Fpr2/3, as a relay mechanism, to control the extent of the host vascular response (Figure 6). In favor of this mechanism is the notion that ischemia can indeed activate circulating neutrophils, as shown a few years ago with rat CD11b expression in myocardial infarct.12 This cell type will become fully activated following reperfusion.25,38 We cannot exclude the notion that other endogenous Fpr2/3 agonists could be involved in this initial response. Potential candidates could be neutrophil-derived peptides generated from cathelicidin39 or systemically generated ligands such as humanin.8 Future studies will investigate this aspect.
The MS results validated the changes in plasma LXA4 during ischemia and provided a degree of specificity because levels of cognate bioactive lipids such as LXB4 or LTB4 were unaltered. Analysis of HETE species revealed augmented levels of 15-HETE in ischemia over sham animals, yet this change occurred in both genotypes to an equal extent. This result indicates that the low LXA4 levels we quantified in ischemic plasma of Fpr2/3−/− mice are not due to enzymatic failure of the biosynthetic pathway, but rather to inadequate formation of platelet/PMN aggregates.
We concluded this series of experiments by testing whether this novel circuit could be activated by ASA. This compound can activate multiple mechanisms besides cyclo-oxygenase inhibition, including interference with transcription factor or adenosine signaling.40 ASA is known to trigger generation of 15-epi-LXA4 after acetylation of cyclo-oxygenase 2 catalytic site22,24 and can also augment native LXA4 synthesis by shunting arachidonate metabolism toward the 5-lipoxygenase pathway.6 In any case, ASA can exert anti-inflammatory properties via LXA4 generation20 as shown in experimental settings and confirmed in human investigational studies,41,42 yet reliance on endogenous LXA4 by ASA in ischemia has scarcely been studied. A recent investigation in patients undergoing abdominal aortic aneurysm surgery revealed lipid-based stratification between pro-inflammatory and pro-resolving phenotypes.43 Here we compared ASA with indomethacin, another cyclo-oxygenase inhibitor unable to shunt of the catalytic activity of the enzyme. Application of full dose-responses revealed that ASA could jumpstart the mechanism we have uncovered here, by initiating 15-epi-LXA4 synthesis, which would then act on Fpr2/37,11 to inhibit the post-reflow inflammatory events within the mesenteric microcirculation (Figure 6). At the highest doses tested, ASA acted in an Fpr2/3-independent fashion, likely by altering the generation of prostanoids and/or engaging multiple disparate pathways.44 We would like to propose that similar events may underlie the efficacy of statins in IR settings, because these drugs can trigger 15-epi-LXA4 synthesis after cysteine S-nitrosylation of cyclo-oxygenase-2,45 reviewed previously.46
In conclusion, neutrophil Fpr2/3 fulfils a crucial function in vascular inflammation. Besides transducing the pharmacological properties of LXA4 and AnxA1 in ischemia-perfusion injury,10 this homeostatic G protein–coupled receptor becomes engaged during the early ischemic phase by governing the interaction between circulating neutrophils and platelet (Figure 6). Platelet/neutrophil aggregates peak at the occlusion of the superior mesenteric artery and predispose to a “proper controlled” vascular inflammatory response that subsides within 2 to 3 hours. In the absence of Fpr2/3, an aberrant inflammatory response takes place because of, at least in part, the inadequate production of LXA4 that is characterized by prolonged neutrophil adhesiveness and infiltration. It is plausible that this vasculoprotective circuit we describe here might be operative in other ischemic events in which rapid generation of LXA4 occurs to avoid harmful overshooting of the host response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Wellcome Trust (program 086867/Z/08/Z) and forms part of the research themes contributing to the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, which is supported and funded by the National Institutes of Health Research.
Wellcome Trust
Authorship
Contribution: V.B. performed experiments, analyzed data, and wrote the first draft of the manuscript; T.G., N.C., P.l.F., and B.C. performed experiments and analyzed data; R.J.F., N.V., and S.N. planned the experiments and revised the manuscript; and M.P. planned the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for V.B. is Department of Science, University of Basilicata, Potenza, Italy.
Correspondence: Mauro Perretti, The William Harvey Research Institute, Barts and The London School of Medicine, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; e-mail: m.perretti@qmul.ac.uk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal