Key Points
PGN forms immune complexes with preexisting human anti-PGN antibodies to activate the classical complement pathway.
Human platelets are activated by PGN–anti-PGN immune complexes through platelet FcγRIIa and through platelet binding C5b.
Abstract
Platelet activation frequently accompanies sepsis and contributes to the sepsis-associated vascular leakage and coagulation dysfunction. Our previous work has implicated peptidoglycan (PGN) as an agent causing systemic inflammation in gram-positive sepsis. We used flow cytometry and fluorescent microscopy to define the effects of PGN on the activation of human platelets. PGN induced platelet aggregation, expression of the activated form of integrin αIIbβ3, and exposure of phosphatidylserine (PS). These changes were dependent on immunoglobulin G and were attenuated by the Fcγ receptor IIa–blocking antibody IV.3, suggesting they are mediated by PGN–anti-PGN immune complexes signaling through Fcγ receptor IIa. PS exposure was not blocked by IV.3 but was sensitive to inhibitors of complement activation. PGN was a potent activator of the complement cascade in human plasma and caused deposition of C5b-9 on the platelet surface. Platelets with exposed PS had greatly accelerated prothrombinase activity. We conclude that PGN derived from gram-positive bacteria is a potent platelet agonist when complexed with anti-PGN antibody and could contribute to the coagulation dysfunction accompanying gram-positive infections.
Introduction
In humans, sepsis is caused by an exaggerated inflammatory response to components of infectious organisms. The inflammation involves platelets which propagate blood coagulation in the vasculature. Thrombocytopenia resulting from in vivo platelet aggregation is common in patients in intensive care units with bacterially driven sepsis1 and is prognostic for intensive care unit mortality.2 Thrombocytopenia is associated with changes in vascular permeability,3 a common feature in sepsis.3 Furthermore, platelet aggregation contributes to the coagulation dysfunction found in severe sepsis by providing a surface for the propagation of blood coagulation through binding of coagulation factors V and X4 to promote pathological thrombus formation in the vasculature and organs.
The proximal cause of systemic inflammation in gram-negative infections is lipopolysaccharide. However, a corresponding gram-positive bacterial product that causes systemic inflammation and can activate platelets is less well defined. The cell walls of gram-positive bacteria are polymers of peptidoglycan (PGN), teichoic acids, and palmitoylated proteins.5 Many of these polymers are recognized by Toll-like receptors (TLRs) and/or nucleotide-binding oligomerization domain (NOD) sensors expressed by immune cells.6 PGN, a disaccharide polymer highly crosslinked by a short peptide chain, is the core component of gram-positive cell walls. The minimal essential structures of PGN, isoglutamine-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), are detected by the cytoplasmic NOD17 and 28 receptors.
Whether and if mammalian immune cells recognize PGN has been controversial. Earlier studies concluded that PGN stimulated TLR29 but more recent studies showed that TLR2 recognition of PGN was instead due to contaminants in PGN preparations.10,11 Our previous work established that extensively purified polymeric PGN stimulates proinflammatory cytokines from innate immune cells12-14 by phagocytosis and digestion in lysosomes to the iE-DAP and MDP monomers.13 We also identified the unusual means by which PGN carries out this response: PGN is opsonized by anti-PGN immunoglobulin G (IgG), ubiquitously present in healthy humans.15 The IgG-opsonized PGN binds Fcγ receptors (FcγR) on monocytes and neutrophils to initiate phagocytosis and lysosomal digestion.13,15 Laboratory mice lack anti-PGN IgG15 and hence murine innate immune cells do not respond to PGN by proinflammatory cytokine production.14 Thus, the presence of anti-PGN IgG and the expression of a FcγR are necessary for innate immune cells to respond to PGN.
Human platelets,16 but not those of rodents, express FcγRIIa, an activating IgG receptor. Binding of platelet FcγRIIa to IgG-opsonized targets induces platelet activation, indicated by platelet aggregation, expression of activated integrin αIIbβ3 capable of fibrinogen binding,17 and exposure of phosphatidylserine (PS).18 These events are induced by a FcγRIIa-triggered activation of Src-family and Syk tyrosine kinases that induce tyrosine phosphorylation of cytoplasmic targets.19,20 Gram-positive bacteria are known to activate human platelets in an IgG-dependent manner,21,22 but specific antibody targets within bacteria are largely undefined. It is possible that PGN–anti-PGN immune complexes contribute to the thrombocytopenia and platelet aggregation seen in gram-positive sepsis.
Nonhuman primates challenged in vivo with the gram-positive organism Bacillus anthracis show many of the clinical features of sepsis, including platelet loss and vascular leakage.23 Here, we tested the activation of human platelets by PGN derived from B anthracis. We show that PGN induces platelet activation, indicated by platelet aggregation, integrin activation, and PS exposure. Aggregation and expression of activated integrin αIIbβ3 required anti-PGN IgG and FcγRIIa. PS exposure was a function of anti-PGN IgG but did not require FcγR engagement. Rather, PS exposure was due to activation of the complement cascade by PGN–anti-PGN immune complexes, leading to the formation of C5b-9 membrane attack complex and binding to platelets. Together, our findings identify 2 mechanisms by which PGN–anti-PGN immune complexes are able to activate human platelets: directly through FcγR and indirectly through C5b-9. The observations indicate that PGN may promote the blood coagulation that accompanies severe gram-positive infections.
Methods and materials
Materials
Alexa Fluor 647 anti-human CD41 was purchased from BioLegend. PAC-1 FITC and mouse anti-human CD9 were purchased from BD Biosciences. Annexin V–fluorescein isothiocyanate (FITC) was purchased from Sigma. Human IgG was purchased from Lampire Biological Laboratories or was a gift from Grifols Therapeutics. Mouse anti-phosphotyrosine (monoclonal antibody 4G10) was from Millipore. Mouse monoclonal antibodies to C5b-9 (clone aE11) and biotinylated anti-C6 (clone 9C4) were kind gifts of Dr Tom Mollnes (University of Oslo, Oslo, Norway). Compstatin and ML161 were from Tocris Bioscience. Anti-C5 antibody (Soliris; eculizumab) monoclonal antibody was purchased from Alexion Pharmaceuticals. The monoclonal antibody to C5a was a gift from Niels Riedmann (InflaRx GmbH, Jena, Germany). Bovine coagulation factors were kind gifts of Dr C. T. Esmon (Oklahoma Medical Research Foundation, Oklahoma City, OK).
Blood collection, platelet-rich plasma preparation, and platelet isolation
Blood and blood products were purchased from the Oklahoma Blood Institute (Oklahoma City, OK) or obtained from local volunteers after informed consent in accordance with the Declaration of Helsinki. The work was approved by the institutional review board. Peripheral blood was collected according to the approved venipuncture protocol into 3.8% sodium citrate to collect plasma or with no anticoagulant to collect human serum. Platelet-rich plasma was prepared by centrifugation of whole blood at 180g for 7 minutes at room temperature. Platelets and platelet-poor plasma were centrifuged at 2000g for separation. Platelets were washed and suspended in Tyrode buffer.
IgG removal from plasma and serum
Human serum was incubated with protein G magnetic microbeads (Miltenyi Biotec) for 1 hour at room temperature. After the beads were removed by a magnet, the IgG concentration of the plasma was reduced 20-fold, as determined by quantitative serial dilutions and immunoblotting with anti-human IgG antibodies. Anti-PGN IgG was removed from plasma by incubation of 0.5 mL of plasma with 100 μg of PGN for 1 hour at 4°C followed by centrifugation to remove the PGN as described.15 When indicated, IgG was restored to a concentration of 500 μg/mL using IVIg (Grifols Therapeutics).
Platelet aggregation
Platelet-rich plasma was mixed with PGN, prepared as described,13-15 or plasma-opsonized PGN at 37°C and light transmission was measured with a PAP-4 Bio/Data 4-chamber aggregometer (Bio/Data Corporation) in the presence of 2.5 mM CaCl2. For some experiments, PGN was preopsonized with plasma or serum overnight at 4°C; plasma and serum were equally effective. In such cases, the final concentration of PGN was always 10 μg/mL and the final amount of plasma/serum was 10% v/v.
Flow cytometric studies of platelets
Isolated platelets at 1-5 × 106/mL in Tyrode buffer containing 3 mM Ca+2 were incubated with stirring at 1200 rpm with thrombin (1 IU/ml), PGN (10 μg/mL) or PGN preincubated in plasma overnight at 4°C for 15 minutes. When noted, we added anti-FcγR monoclonal antibody IV.3 (10 μg/mL), Triofiban to 1 µM, ML161 to 100 µM, and Arg-Gly-Asp-Ser (Sigma) to 100 µM. Before analysis, all samples were filtered through 80-μm nylon mesh to remove aggregated platelets. Platelets were labeled with Cy5–anti-human CD41 with or without FITC-annexin V or FITC–PAC-1 antibody, then fixed with 1% formalin. Flow cytometry was performed on a FACSCalibur; gating was performed on forward and side scatter and CD41 to identify platelets (supplemental Figure 1, available on the Blood website). The results were analyzed by FloJo software (TreeStar Inc).
Confocal microscopy
Isolated platelets were treated with 10 μg/mL PGN preincubated with plasma at 37°C for 1 hour. The platelets were stained with Cy5–anti-human CD41 and FITC–annexin V or FITC–anti-human C5b-9 antibody (aE11). After the platelets were fixed in 2% formaldehyde, the cells were spread on poly-lysine–coated glass slides and observed with a Zeiss LSM 510 confocal microscope.
ELISA of C5b-9
Human sera depleted of C1q, Factor D, IgG, or C8 was purchased from Complement Technology, Inc. Neutralizing anti-MBL antibodies (Hycult Biotechnology) were added to a final concentration of 10 µg/mL. EDTA and EGTA were used at 10 mM. PGN (10 μg/mL) at 37°C was added to human sera for 1 hour and the reaction was stopped by 10 mM EDTA. Complement products were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) as previously described24,25 using aE11 to capture C5b-9 and detecting with biotinylated anti-human C6.
Prothrombinase assay
Platelets were washed and diluted in assay buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid], 137 mM NaCl, 4 mM KCl, 2.5 mM CaCl2, 5 mM glucose, and 0.5% bovine serum albumin, pH 7.4). Platelets (∼1 × 105) were seeded into a 96-well plate and left unstimulated or stimulated with ionomycin (10 µM), PGN (10 µg/mL), or PGN preincubated with normal plasma (final concentration, 10%). Following stimulation, a cocktail containing bovine coagulation factors fVa (10 nM final concentration), fXa (1 nM) prothrombin (200 nM), and the substrate Spectrozyme TH (Sekisui Diagnostics; 0.5 mM) were added to each well, and the conversion of the chromogenic substrate was measured kinetically every minute for 1 hour at 405 nm. Substrate conversion as a function of time was averaged from 3 independent measurements per condition and is depicted after normalization to the maximum substrate conversion defined as 100%.
Statistical analysis
Statistical analyses were performed with GraphPad Prism software. Statistical significance was determined using a t test and a P value of < .05 was considered statistically significant.
Results
PGN from gram-positive bacteria activates platelets
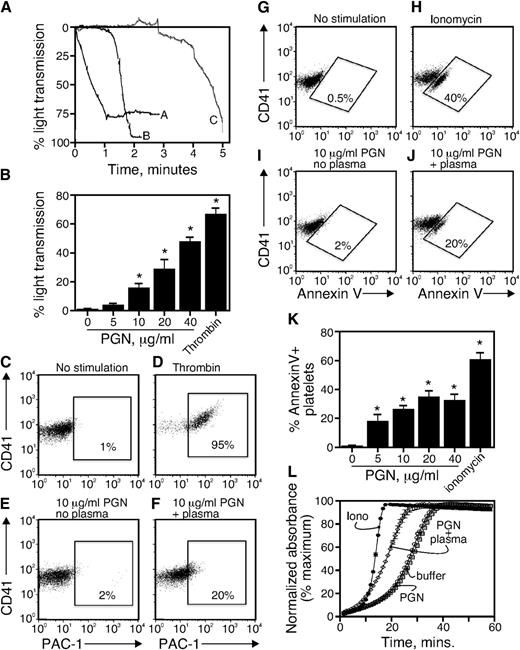
We added PGN at 37°C to platelet-rich plasma obtained from normal subjects and recorded changes in light transmission over time as an indicator of platelet aggregation. We found that, like thrombin stimulation (Figure 1A, line A), PGN induced aggregation of normal platelets (Figure 1A, line C). However, while thrombin-induced platelet aggregation was immediate, PGN-induced aggregation occurred after a lag time of between 3 and 5 minutes which varied with the platelet donor. PGN preincubated with normal human plasma (1 hour at 37°C then stored at 4°C) induced platelet aggregation with a reduced lag time (Figure 1A, line B). We measured platelet aggregation after a 15-minute incubation with varying concentrations of PGN (1-40 μg/mL) preincubated with normal human plasma. Although higher PGN doses (Figure 1B) and/or longer incubation times (not shown) produced a stronger platelet response, flow cytometry–based experiments became difficult as platelets became increasingly aggregated. To obtain a robust platelet response that still permitted flow cytometry assays, for the remaining experiments, we preincubated 10 μg/mL PGN with plasma overnight at 4°C followed by a 15-minute incubation with platelets at 37°C unless otherwise indicated.
PGN from gram-positive bacteria activates human platelets. (A) Aggregation traces for (line A) platelet-rich plasma + thrombin (1 U/mL); (line B) PGN preincubated in plasma for 30 minutes at 37°C and then added to platelet-rich plasma; (line C) platelet-rich plasma + PGN (10 μg/mL). For line C, the final concentration of PGN was 10 μg/mL and of plasma was 10% v/v. (B) PGN (0-40 μg/mL) preincubated with plasma as described in panel A or thrombin (1 U/μλ) was added to platelets, and percentage of light transmission was monitored for 15 minutes. Data are reported as means of the percentage of light transmission of 4 independent experiments from different donors. (C-J) Washed platelets were stimulated with (C,G) Tyrode buffer, (D) thrombin, (H) ionomycin, (E,I) 10 μg/mL PGN in Tyrode buffer, and (F,J) 10 μg/mL PGN preincubated with plasma at 37°C for 15 minutes. After fixation, platelets were identified by CD41 and analyzed (C-F) for αIIbβ3 upregulation by PAC-1 or (G-J) PS exposure by annexin V. The data in C through J are representative of >10 experiments. (K) Platelet-rich plasma was pretreated with tirofiban (1 μM) to prevent platelet aggregation. PGN or ionomycin (10 μM) were added to platelet-rich plasma for 30 minutes at 37°C. After fixing and staining, annexin V–positive platelets were examined by flow cytometry. (L) The exposure of anionic phospholipids on the platelet surface was analyzed by a continuous prothrombinase activity assay. Platelets were incubated with buffer (open circles), 10 μg/mL PGN (squares), plasma-preincubated PGN (diamonds), or 10 μM ionomycin (closed circles). Thrombin generation was followed over time using the chromogenic substrate Spectrozyme TH. A representative experiment performed in triplicate is shown after normalization to the maximum conversion of the substrate.
PGN from gram-positive bacteria activates human platelets. (A) Aggregation traces for (line A) platelet-rich plasma + thrombin (1 U/mL); (line B) PGN preincubated in plasma for 30 minutes at 37°C and then added to platelet-rich plasma; (line C) platelet-rich plasma + PGN (10 μg/mL). For line C, the final concentration of PGN was 10 μg/mL and of plasma was 10% v/v. (B) PGN (0-40 μg/mL) preincubated with plasma as described in panel A or thrombin (1 U/μλ) was added to platelets, and percentage of light transmission was monitored for 15 minutes. Data are reported as means of the percentage of light transmission of 4 independent experiments from different donors. (C-J) Washed platelets were stimulated with (C,G) Tyrode buffer, (D) thrombin, (H) ionomycin, (E,I) 10 μg/mL PGN in Tyrode buffer, and (F,J) 10 μg/mL PGN preincubated with plasma at 37°C for 15 minutes. After fixation, platelets were identified by CD41 and analyzed (C-F) for αIIbβ3 upregulation by PAC-1 or (G-J) PS exposure by annexin V. The data in C through J are representative of >10 experiments. (K) Platelet-rich plasma was pretreated with tirofiban (1 μM) to prevent platelet aggregation. PGN or ionomycin (10 μM) were added to platelet-rich plasma for 30 minutes at 37°C. After fixing and staining, annexin V–positive platelets were examined by flow cytometry. (L) The exposure of anionic phospholipids on the platelet surface was analyzed by a continuous prothrombinase activity assay. Platelets were incubated with buffer (open circles), 10 μg/mL PGN (squares), plasma-preincubated PGN (diamonds), or 10 μM ionomycin (closed circles). Thrombin generation was followed over time using the chromogenic substrate Spectrozyme TH. A representative experiment performed in triplicate is shown after normalization to the maximum conversion of the substrate.
We used flow cytometry to test the effects of PGN on integrin αIIbβ3 and PS exposure in the absence and presence of plasma. After a 15-minute incubation under the given conditions, platelets were stained with the antibody PAC-1 to detect activated integrin αIIbβ3 or annexin V to detect PS exposure. The positive controls thrombin (Figure 1D) and ionomycin (Figure 1H) were potent inducers of integrin αIIbβ3 and PS exposure, respectively. In the absence of human plasma, PGN failed to induce integrin αIIbβ3 activation or PS exposure (Figure 1E,I) while PGN preincubated with plasma caused a significant increase in both events (Figure 1F,J). We measured PS exposure in response to varying doses of PGN when aggregation was blocked by tirofiban, a nonpeptide antagonist of integrin αIIbβ3 function.26 Tirofiban completely blocked aggregation induced by PGN or thrombin (supplemental Figure 2) and allowed us to measure PGN-induced PS exposure on platelets according to the PGN dose (Figure 1K). Aggregation and PS exposure in platelets was not affected by ML161, an allosteric inhibitor of protease-activated receptor-1,27 nor was PS exposure affected by platelet concentration (supplemental Figures 2-4). Lastly, we used a thrombin-specific chromogenic substrate Spectrozyme TH to measure prothrombinase activity on platelets. We found (Figure 1L) that PGN enhanced prothrombinase activity only in the presence of human plasma. Together, these results indicate that PGN-induced platelet activation requires factors present in human plasma.
Both PGN and plasma factors contribute to platelet activation
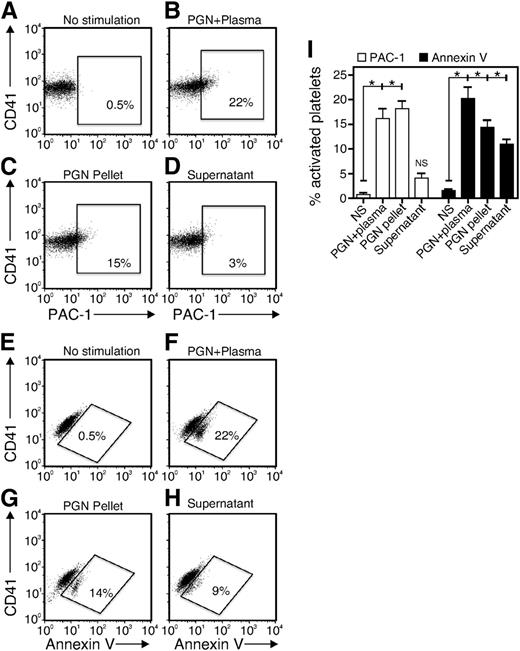
To test plasma-mediated opsonization, we preincubated PGN in human plasma for 1 hour at 37°C and centrifuged the mixture to separate the PGN-containing pellet and the remaining plasma factors in the supernatant. The pellet and supernatant were used separately to stimulate platelets. For comparison, we stimulated platelets with 10 μg/mL PGN preincubated with plasma for 1 hour at 37°C as described in the first paragraph. (PGN + plasma; Figure 2B,F). The PGN pellet at 10 μg/mL induced significant expression of activated integrin (PGN pellet; Figure 2C,I) while the plasma factors remaining in the supernatant failed to induce integrin activation (supernatant; Figure 2D,I). In contrast, both the PGN pellet and the supernatant were able to trigger PS exposure on platelets (Figure 2G-I), although both at lower levels than whole plasma (Figure 2F). These results suggest that PGN-induced platelet activation requires opsonization of PGN by factors in the plasma. However, the results also suggest that PGN-induced integrin αIIbβ3 activation and PS exposure occur through distinct mechanisms.
Both PGN and plasma factors contribute to platelet activation. PGN was preincubated in plasma for 1 hour and then centrifuged at 2000g to separate the particulate PGN pellet and PGN-activated plasma supernatant. The washed platelets were stimulated independently with PGN preincubated with plasma (PGN + Plasma, B and F), the PGN pellet (PGN Pellet, C and G) or PGN-activated plasma supernatant (Supernatant, D and H) before staining for (A-D) PAC-1 or (E-H) annexin V and analysis by flow cytometry. (I) Quantitative and statistical data are shown for 3 replicates of experiments performed as in panels A through H. *P < .05 and NS, not significant for comparisons of no stimulation (NS) with PGN + plasma, PGN pellet, or supernatant.
Both PGN and plasma factors contribute to platelet activation. PGN was preincubated in plasma for 1 hour and then centrifuged at 2000g to separate the particulate PGN pellet and PGN-activated plasma supernatant. The washed platelets were stimulated independently with PGN preincubated with plasma (PGN + Plasma, B and F), the PGN pellet (PGN Pellet, C and G) or PGN-activated plasma supernatant (Supernatant, D and H) before staining for (A-D) PAC-1 or (E-H) annexin V and analysis by flow cytometry. (I) Quantitative and statistical data are shown for 3 replicates of experiments performed as in panels A through H. *P < .05 and NS, not significant for comparisons of no stimulation (NS) with PGN + plasma, PGN pellet, or supernatant.
The amount of plasma is rate limiting for platelet activation
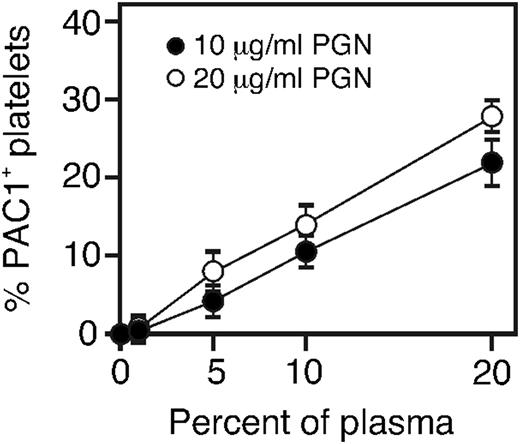
To better understand the plasma-derived factors that might contribute to PGN-induced platelet activation, we assessed levels of activated integrin αIIbβ3 in platelets incubated with 10 or 20 μg/mL PGN preopsonized with various amounts of human plasma. Doubling the amount of PGN from 10 to 20 μg/mL only slightly increased the platelet response (Figure 3). In contrast, doubling the amount of plasma doubled the response to either dose of PGN. Thus, the extent of the platelet response to PGN is limited by the amount of factors in human plasma, and less so by the amount of PGN. Our previous work demonstrated that all normal healthy donors contained IgG anti-PGN antibodies which were required to stimulate an inflammatory response via FcγR in human innate immune cells.15 Therefore, we hypothesized that IgG plays a role in PGN-stimulated platelet activation.
Plasma and not PGN is limiting for platelet activation. PGN (10 or 20 μg/mL final concentrations) was incubated with the indicated amount of plasma (final concentrations, v/v) for 30 minutes at 37°C before adding to washed platelets for 15 minutes. Platelets were fixed, and the percent of PAC-1–expressing platelets was determined by flow cytometry. The data are the average from 3 independent experiments.
Plasma and not PGN is limiting for platelet activation. PGN (10 or 20 μg/mL final concentrations) was incubated with the indicated amount of plasma (final concentrations, v/v) for 30 minutes at 37°C before adding to washed platelets for 15 minutes. Platelets were fixed, and the percent of PAC-1–expressing platelets was determined by flow cytometry. The data are the average from 3 independent experiments.
PGN binding to and stimulation of platelet requires IgG
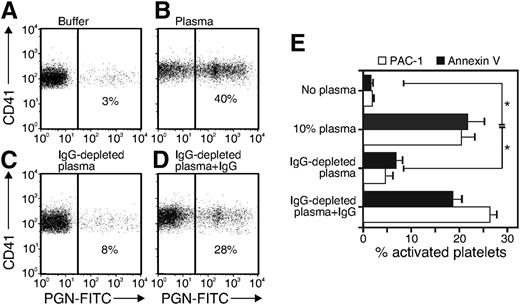
We tested platelet-PGN binding using FITC-labeled PGN combined with flow cytometry when aggregation was blocked by tirofiban. Consistent with the observation that PGN-induced platelet activation required plasma, PGN binding to platelets was minimal in the absence of plasma (Figure 4A) while 40% of platelets bound FITC-PGN that had been preincubated with plasma (Figure 4B). Only 8% of platelets bound PGN that had been incubated in IgG-depleted plasma (Figure 4C) and reconstituting IgG-depleted plasma with normal human IgG restored PGN-FITC binding (Figure 4D). Similarly, platelets showed activation of integrin αIIbβ3 and PS exposure after stimulation with 10 μg/mL PGN preincubated with normal human plasma or IgG-reconstituted plasma but only minimal responses when PGN was preincubated with IgG-depleted plasma (Figure 4E). Thus, IgG is required for PGN-triggered expression of the activated integrin αIIbβ3 and for elevated PS exposure, consistent with our previous findings on human monocytes and neutrophils.15
PGN binding to and stimulation of platelets requires IgG. PGN-FITC was incubated with Tyrode buffer (buffer), human plasma (plasma), Ig-depleted plasma, or IgG-depleted plasma + 500 μg/mL human IgG. Platelets were washed free of plasma and incubated in the presence of 1 µM tirofiban with the PGN opsonized with plasma for 30 minutes before staining for Cy5-CD41. The percentage of CD41+ platelets binding to PGN-FITC was determined by flow cytometry. The result is representative of 4 experiments. (E) Platelets were prepared and treated with PGN opsonized as described in panels A through D. The activation of platelets was detected by staining with PAC-1 or annexin V. The data are the mean and SE of four experiments, each with a different donor for cells and plasma. *P < .05.
PGN binding to and stimulation of platelets requires IgG. PGN-FITC was incubated with Tyrode buffer (buffer), human plasma (plasma), Ig-depleted plasma, or IgG-depleted plasma + 500 μg/mL human IgG. Platelets were washed free of plasma and incubated in the presence of 1 µM tirofiban with the PGN opsonized with plasma for 30 minutes before staining for Cy5-CD41. The percentage of CD41+ platelets binding to PGN-FITC was determined by flow cytometry. The result is representative of 4 experiments. (E) Platelets were prepared and treated with PGN opsonized as described in panels A through D. The activation of platelets was detected by staining with PAC-1 or annexin V. The data are the mean and SE of four experiments, each with a different donor for cells and plasma. *P < .05.
FcγRII binding is required for PGN induced integrin αIIbβ3 activation but not PS exposure
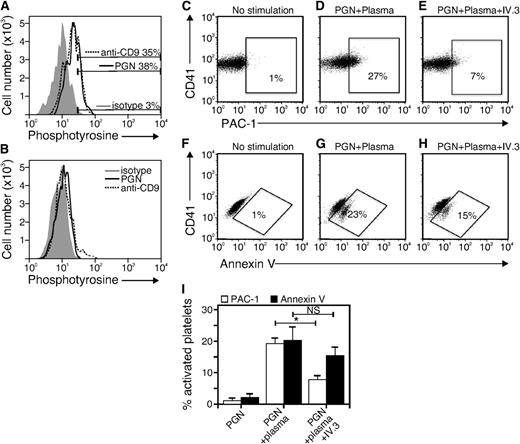
The data in Figure 4 raise the possibility that IgG-opsonized PGN engages platelet FcγRIIa to induce integrin αIIbβ3 activation and PS exposure. Because FcγRIIa-stimulated platelets show tyrosine phosphorylation of cytoplasmic proteins, we evaluated the presence of tyrosine-phosphorylated proteins in PGN-stimulated platelets. As a positive control, we stimulated platelets with antibodies to the platelet surface protein CD9, as anti-CD9 antibody is known to engage platelet FcγRIIa to stimulate intracellular tyrosine phosphorylation.28 After incubation with anti-CD9 or with PGN preincubated with plasma, platelets were fixed, permeabilized, stained with anti-phosphotyrosine antibody, and analyzed by flow cytometry. Both PGN (Figure 5A, solid line) and anti-CD9 (Figure 5A, dotted line) induced an increase in cytoplasmic tyrosine-phosphorylated proteins. To ensure the specificity of anti-phosphotyrosine antibody staining, we repeated the experiment but stained the cells with anti-phosphotyrosine in the presence of free phosphotyrosine. This competition assay confirmed the specificity of the anti-phosphotyrosine antibody as the signal for PGN and anti-CD9 were at background (Figure 5B). These findings are consistent with the hypothesis that IgG-opsonized PGN engages platelet FcγRIIa.
FcγRII binding is required for PGN-induced integrin αIIbβ3 upregulation but not PS exposure. Platelet-rich plasma was stimulated with PGN (10 μg/mL) or anti-CD9 (10 μg/mL) at 37°C for 30 minutes. After fixation, platelets were permeabilized with saponin and stained with anti-phosphotyrosine antibody in the (A) absence or (B) presence of 10 mM free phosphotyrosine. (C-H) Flow cytometry analysis of PAC-1 staining (C-E) and annexin V staining (F-H) using washed platelets suspended in Tyrode buffer. Platelets were stimulated with nothing (no stimulation) or PGN preincubated with plasma for 30 minutes at 37°C (PGN + Plasma). Stimulation was done in the presence or absence of anti-human FcγRII blocking Abs (IV.3). (I) Quantitative and statistical data of 3 similar experiments. *P < .05; NS, not significant.
FcγRII binding is required for PGN-induced integrin αIIbβ3 upregulation but not PS exposure. Platelet-rich plasma was stimulated with PGN (10 μg/mL) or anti-CD9 (10 μg/mL) at 37°C for 30 minutes. After fixation, platelets were permeabilized with saponin and stained with anti-phosphotyrosine antibody in the (A) absence or (B) presence of 10 mM free phosphotyrosine. (C-H) Flow cytometry analysis of PAC-1 staining (C-E) and annexin V staining (F-H) using washed platelets suspended in Tyrode buffer. Platelets were stimulated with nothing (no stimulation) or PGN preincubated with plasma for 30 minutes at 37°C (PGN + Plasma). Stimulation was done in the presence or absence of anti-human FcγRII blocking Abs (IV.3). (I) Quantitative and statistical data of 3 similar experiments. *P < .05; NS, not significant.
As a more definitive test of FcγRIIa involvement, we used the monoclonal anti-FcγRIIa–specific antibody IV.3 to block immune complex binding. As expected, platelets that were stimulated with 10 μg/mL PGN preincubated with plasma showed increased active integrin αIIbβ3 epitope (Figure 5D) and PS exposure (Figure 5G). Integrin activation was prevented by inclusion of the FcγRIIa-blocking antibody IV.3 (Figure 5E,I). Additionally, PGN-induced activation of αIIbβ3 was completely blocked by 10 μM piceatannol, a Syk kinase inhibitor, and was accompanied by Syk tyrosine phosphorylation that was blocked by inclusion of IV.3 (supplemental Figure 4), events we showed earlier in FcγRIIA-stimulated platelets.19,20 PS exposure was also somewhat reduced in the presence of IV.3 (Figure 5H-I) but the difference was not statistically significant. These findings establish that the PGN–anti-PGN immune complexes bind to platelet FcγRIIa to stimulate integrin αIIbβ3 upregulation and suggest that PS exposure is triggered by another mechanism.
PGN uses the complement pathway to induce platelet PS exposure
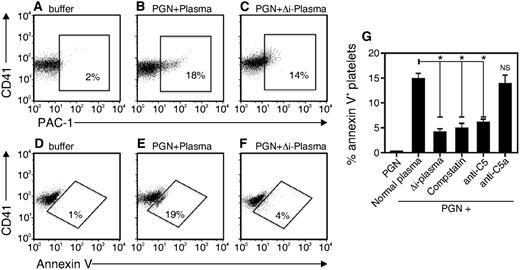
Because PGN-triggered PS exposure in platelets required IgG (Figure 4) but was independent of FcγRIIa (Figure 5H), we hypothesized that PGN-induced PS exposure might be the result of complement activation. To test the role complement activation, we incubated platelets with 10 μg/mL PGN in the presence of buffer, normal plasma, or plasma in which complement was heat-inactivated (Δi-plasma). Compared with normal plasma (Figure 6B,E), pretreatment with heat-inactivated plasma only slightly decreased platelet expression of activated integrin αIIbβ3 (Figure 6C) but dramatically reduced the exposure of PS (Figure 6F). The data are consistent with a need for complement activation in human platelets for PGN-induced PS exposure but not for expression of the activated αIIbβ3 integrin.
PS exposure in human platelets requires complement activation. PGN was preincubated for 30 minutes at 37°C with Tyrode buffer (buffer), normal plasma (PGN + plasma) or heat-inactivated plasma (PGN + Δi Plasma) and then added to platelets for 15 minutes at 37°C. Integrin αIIbβ3 and PS expression were detected with (A-C) PAC-1 antibody or (D-F) annexin V, respectively. (G) Normal plasma was pretreated with compstatin, anti-human C5, or C5a (10 μg/mL) for 1 hour before mixing with PGN for 1 hour at 37°C. The mixture was added to platelets in Tyrode buffer and the percentage of annexin V–positive platelets after 15 minutes at 37°C was recorded. The data are representative of 3 independent experiments. *P < .05.
PS exposure in human platelets requires complement activation. PGN was preincubated for 30 minutes at 37°C with Tyrode buffer (buffer), normal plasma (PGN + plasma) or heat-inactivated plasma (PGN + Δi Plasma) and then added to platelets for 15 minutes at 37°C. Integrin αIIbβ3 and PS expression were detected with (A-C) PAC-1 antibody or (D-F) annexin V, respectively. (G) Normal plasma was pretreated with compstatin, anti-human C5, or C5a (10 μg/mL) for 1 hour before mixing with PGN for 1 hour at 37°C. The mixture was added to platelets in Tyrode buffer and the percentage of annexin V–positive platelets after 15 minutes at 37°C was recorded. The data are representative of 3 independent experiments. *P < .05.
To more precisely define the complement component(s) that stimulates PS exposure on platelets, we added various complement inhibitors during the pretreatment of PGN with plasma. The results of several experiments are summarized in Figure 6G. Compstatin, a C3 convertase inhibitor,29 blocked PS exposure as efficiently as heat inactivation of plasma. Neutralizing antibodies to C5 likewise prevented PS exposure on platelets, indicating the active complement component is formed downstream of C5. However, neutralizing antibodies to C5a did not affect PS exposure, showing that the active complement component triggering PS exposure on platelets is not the anaphylotoxin C5a. Together, these data suggest that PGN-induced PS exposure on platelets is stimulated by the terminal attack complex formed distal to the conversion of C5.
PGN activates the complement cascade in human sera
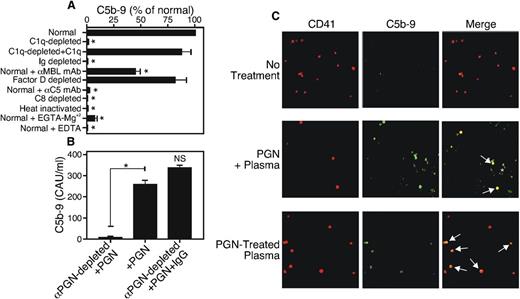
We used aE11, a monoclonal antibody that detects a nascent epitope of C9 present in the terminal attack complex,30 in an ELISA to test whether PGN induces the formation of C5b-9. We found that 10 μg/mL PGN added directly to normal human serum was a potent inducer of C5b-9 (Figure 7A), a process that was heat-sensitive and was blocked in the absence of components of the common activation pathway (C5 or C8) or by Ca+2 chelation with EDTA. Complement activation was maximal when IgG and C1q were present, and it was inhibited by EGTA-Mg+2, indicating activation of the classical pathway. Adding back-purified C1q restored PGN-triggered C5b-9 formation. Incubation with an inhibitory monoclonal antibody to MBL led to partial inhibition of C5b-9 generation, suggesting that the lectin pathway can also support PGN-induced complement activation. The alternative pathway did not make a significant contribution to PGN-driven complement activation as C5b-9 formation was not reduced in sera depleted of Factor D. We repeated the experiment using sera that had been depleted of anti-PGN antibodies by incubation with PGN particles at 4°C. Anti-PGN–depleted sera failed to activate complement upon PGN addition, while IgG-reconstituted sera responded to PGN addition by complement activation (Figure 7B). These data strongly suggest that PGN–anti-PGN immune complexes activate the complement cascade, primarily through the classical pathway.
PGN–anti-PGN immune complexes activate the classical complement pathway to induce C5b-9 complex formation. (A) Normal serum, Ig-depleted serum, commercial serum that was C1q-, C8-, or Factor d–depleted, or serum to which 10 µg/mL neutralizing antibodies to C5 or MBL were added, was incubated with 10 μg/mL PGN at 37°C for 1 hour, and the reaction was stopped by 10 mM EDTA. The serum was added to wells of an ELISA plate precoated with aE11 antibody to capture activated C5b-9. After washing, C5b-9 was detected using biotin–anti-human C6 (clone 9C4) and streptavidin-HRP. (B) Plasma (100 μL) was depleted of anti-PGN antibodies by incubation with PGN (10 μg) overnight at 4°C and the PGN–anti-PGN immune complexes were removed by centrifugation. The supernatant was reconstituted or not with normal human IgG (500 µg/mL), and C5b-9 formation was determined by ELISA as described in panel A. *P < .05.; NS, not significant. (C) Washed platelets were stimulated with PGN preincubated with plasma (PGN + plasma) or PGN-treated plasma supernatant (PGN-treated plasma) at 37°C for 20 minutes. The cells were fixed, stained with Cy5 anti-human CD41 and FITC anti-human C5b-9 and viewed with a Zeiss LSM 510 confocal microscope at ×100 magnification. Arrows point to colocalized CD41+ platelets and FITC-C9. The results are representative of 3 similar experiments. *P < .05.
PGN–anti-PGN immune complexes activate the classical complement pathway to induce C5b-9 complex formation. (A) Normal serum, Ig-depleted serum, commercial serum that was C1q-, C8-, or Factor d–depleted, or serum to which 10 µg/mL neutralizing antibodies to C5 or MBL were added, was incubated with 10 μg/mL PGN at 37°C for 1 hour, and the reaction was stopped by 10 mM EDTA. The serum was added to wells of an ELISA plate precoated with aE11 antibody to capture activated C5b-9. After washing, C5b-9 was detected using biotin–anti-human C6 (clone 9C4) and streptavidin-HRP. (B) Plasma (100 μL) was depleted of anti-PGN antibodies by incubation with PGN (10 μg) overnight at 4°C and the PGN–anti-PGN immune complexes were removed by centrifugation. The supernatant was reconstituted or not with normal human IgG (500 µg/mL), and C5b-9 formation was determined by ELISA as described in panel A. *P < .05.; NS, not significant. (C) Washed platelets were stimulated with PGN preincubated with plasma (PGN + plasma) or PGN-treated plasma supernatant (PGN-treated plasma) at 37°C for 20 minutes. The cells were fixed, stained with Cy5 anti-human CD41 and FITC anti-human C5b-9 and viewed with a Zeiss LSM 510 confocal microscope at ×100 magnification. Arrows point to colocalized CD41+ platelets and FITC-C9. The results are representative of 3 similar experiments. *P < .05.
To test whether activated complement components bind to platelets, we used confocal fluorescent microscopy to visualize PGN-treated or untreated platelets costained with the aE11 monoclonal antibody and anti-CD41 to identify platelets. Untreated platelets showed no C5b-9 reactivity (Figure 7C, top row) while PGN-treated platelets showed colocalization of C5b-9 and the CD41 platelet marker (Figure 7C, middle row). We also preincubated human plasma with 10 μg/mL PGN at 37°C for 1 hour to activate the complement cascade, then removed the PGN by centrifugation. C5b-9 present in this PGN-activated supernatant was also able to bind platelets, indicated by white arrows in the image (Figure 7C, bottom row). These data show that PGN activates the classical complement pathway and that the resulting terminal attack complex is able to bind to platelets, and furthermore that PGN need not be directly associated with platelets to cause C5b-9 binding.
Discussion
Studies on the biological activity of PGN have been confounded by the presence of other bacterial cell wall constituents, including glycolipids, lipoteichoic acid, and acetylated lipopeptides that stimulate various TLRs.31 Early work indicated that PGN was recognized by TLR29 but later studies revealed that the TLR recognition was due to other cell wall contaminants in the PGN preparations.10,11 Indeed, polymeric PGN was considered to be unable to stimulate innate immune cells10,11 and intestinal epithelial cells.32 Because PGN does not stimulate TLRs and because it causes only minimal pathological changes in mice,33-36 PGN has often been overlooked as a pathogen-associated molecular pattern. However, a recent body of work from our laboratory demonstrates the importance of PGN as a pathogen-associated molecular pattern when anti-PGN IgG is present.15 Unfortunately, mice lack the necessary anti-PGN antibodies,14 which leaves us without an appropriate animal model for in vivo PGN studies. Nevertheless, humans suffer from gram-positive infections where PGN quantities are potentially very large. Such infections can lead to sepsis where thrombocytopenia, coagulation dysfunction, and vascular leakage contribute to the pathology. For these reasons, we evaluated the effects of PGN on human platelets using an in vitro system. Here, we show that human platelets respond to PGN derived from gram-positive bacteria by activation of integrin αIIbβ3 and subsequent platelet aggregation and expression of PS on the cell surface. These events occur through the formation of PGN–anti-PGN immune complexes which stimulate platelet FcγRIIa and activate the complement cascade. The terminal attack complex stimulates PS exposure on platelets which in turn accelerate prothrombinase activity and hence is able to enhance pathological thrombus formation.
Activated platelets release granular contents containing over 400 identified proteins that contribute to the inflammatory process.37 P-selectin is among the granular proteins and its elevated expression on platelets allows binding to neutrophils through neutrophil PSGL-1.38 The resulting platelet-neutrophil complexes are more easily formed in vitro with clinical isolates of gram-positive pathogens,22 where PGN is abundant. The complexes can help clear infections in bacteremic patients by forming neutrophil extracellular traps,39 but also contribute to the disseminated intravascular coagulation by promoting tissue factor expression.40
In addition to directly stimulating platelets via FcγRIIa, PGN–anti-PGN immune complexes indirectly stimulated platelets through complement activation, where C5b-9 binds to platelets leading to PS exposure. PS exposure is a key regulatory event in blood coagulation and is thought to contribute to the thrombotic events that accompany gram-positive sepsis.41 Exposed PS contributes to blood coagulation by binding clotting factors in a calcium-dependent manner42 to allosterically upregulate the activity of the prothrombinase complex (reviewed in Lentz4 ). Indeed, the catalytic efficiency of Factor Xa is increased 300 000-fold on a PS-rich surface.43 Thus, our data suggest that by inducing C5b-9 complex formation, PGN could play a role in microthrombosis and disseminated intravascular coagulation, 2 major contributors to morbidity and mortality in gram-positive sepsis.44
Although PGN may be present at staggering concentrations in humans experiencing severe gram-positive infections, we found that the platelet response was more limited by the availability of plasma factors than by the amount of PGN. Therefore, we hypothesize that the titer of preexisting anti-PGN would be inversely related to the outcome of a gram-positive infection. We previously found the titer of anti-PGN antibody to be quite variable among individuals.15 Our findings predict that individuals with a relatively higher anti-PGN titer would have a more severe response to a gram-positive bacterial infection that would individuals with a relatively lower titer. The observations suggest that F(ab′)2 fragments of anti-PGN IgG might be an effective therapy in gram-positive sepsis because this form of antibody would bind PGN but not engage FcγR nor activate complement.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Drs Clark Anderson (Ohio State University) and J. Donald Capra who read and critiqued the manuscript.
This work was supported by the National Institutes of Health 2 U19 AI062629 (K.M.C.) and GM097747 (F.L.).
Authorship
Contribution: D.S. performed most of the experiments; N.I.P. performed the prothrombinase activity measurements in Figure 1; R.S.K. performed the complement measurements; B.R. prepared and tested the PGN; K.M.C., G.L.D., and F.L. conceived of the experiments; and all authors wrote the paper.
Conflict-of-interest disclosure: Grifols, Inc prepared the IVIg (normal human IgG) which was provided to the authors without cost. The authors are not affiliated in any way with Grifols, Inc and have nothing to disclose.
Correspondence: K. Mark Coggeshall, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK, 73104; e-mail: mark-coggeshall@omrf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal