Key Points
Oxidized LDL stimulates rapid change in platelet shape through ligation of CD36.
Ligation of CD36 by oxidized LDL simultaneously activates tyrosine and Rho kinase–dependent signaling pathways.
Abstract
Oxidized low-density lipoproteins (oxLDL) generated in the hyperlipidemic state may contribute to unregulated platelet activation during thrombosis. Although the ability of oxLDL to activate platelets is established, the underlying signaling mechanisms remain obscure. We show that oxLDL stimulate platelet activation through phosphorylation of the regulatory light chains of the contractile protein myosin IIa (MLC). oxLDL, but not native LDL, induced shape change, spreading, and phosphorylation of MLC (serine 19) through a pathway that was ablated under conditions that blocked CD36 ligation or inhibited Src kinases, suggesting a tyrosine kinase–dependent mechanism. Consistent with this, oxLDL induced tyrosine phosphorylation of a number of proteins including Syk and phospholipase C γ2. Inhibition of Syk, Ca2+ mobilization, and MLC kinase (MLCK) only partially inhibited MLC phosphorylation, suggesting the presence of a second pathway. oxLDL activated RhoA and RhoA kinase (ROCK) to induce inhibitory phosphorylation of MLC phosphatase (MLCP). Moreover, inhibition of Src kinases prevented the activation of RhoA and ROCK, indicating that oxLDL regulates contractile signaling through a tyrosine kinase–dependent pathway that induces MLC phosphorylation through the dual activation of MLCK and inhibition of MLCP. These data reveal new signaling events downstream of CD36 that are critical in promoting platelet aggregation by oxLDL.
Introduction
Blood platelets play an important role in atherothrombosis, the pathologic condition that underpins myocardial infarction and stroke. Hyperlipidemia is a key risk factor for atherothrombosis and is associated with a number of cellular changes including activation of endothelial cells, proliferation of vascular smooth muscle cells, formation of lipid-laden foam cells, and platelet hyperactivity.1,2 The generation of oxidized low-density lipoproteins (oxLDL), a heterogeneous group of modified particles, is intimately linked to hyperlipidemia and is proposed to induce many of the cellular changes observed in atherogenesis. Numerous studies have demonstrated that oxLDL activates blood platelets and potentiates the effect of physiological agonists.3-5 The mechanisms, including receptors and signaling enzymes, underlying these activatory effects are still unclear. To date a number of platelet receptors including integrin αIIbβ3,6 platelet-activating factor receptor,7 scavenger receptor A (SR-A),8 and the class B scavenger receptor CD369 have been identified. Binding of oxLDL to CD36 results in platelet activation through Src kinase and mitogen-activated protein kinase–dependent pathways,9 although signaling through extracellular signal–regulated kinase and focal adhesion-associated kinase may also a play role.8,10 Importantly, CD36 is key to both platelet hyperactivity and accelerated thrombosis in murine models of hyperlipidemia, effects that are mediated via oxidized lipids associated with oxLDL.11 Thus, CD36 plays a prominent role in platelet activation in disease, although the signaling mechanism triggering platelet activity through CD36 remains elusive.
Platelet shape change is the earliest physiological response after activation and is driven by a dynamic remodeling of the actin cytoskeleton. The phosphorylation of regulatory myosin light chains (MLC) on serine 19 of myosin IIA instigates ATPase activity that facilitates myosin interaction with actin filaments.12 This actin-myosin interaction triggers contraction of the actin cytoskeleton required for shape change and granule secretion.13 The phosphorylation state of MLC is determined by the activities of 2 critical regulatory enzymes: MLC kinase (MLCK) and MLC phosphatase (MLCP). MLCK phosphorylates myosin at serine 19 (Ser19), whereas MLCP dephosphorylates this same residue. Platelet agonists stimulate coordinated signaling events that lead to simultaneous activation of MLCK and inhibition of MLCP,14,15 which collectively promote maximal MLC phosphorylation. In human and murine platelets, elevated intracellular Ca2+ in response to numerous platelet agonists activates MLCK through a calmodulin-dependent mechanism,16,17 resulting in the phosphorylation of MLC, shape change, and secretion.16-20 Signaling downstream of G-protein–coupled receptors inhibits MLCP through RhoA-activated Rho kinase (ROCK), which phosphorylates and inhibits MLCP.14 Here we describe novel signaling events where ligation of CD36 by oxLDL triggers 2 Src kinase–dependent pathways, a Src/Syk-dependent and Src/ROCK–dependent pathway, which stimulate platelet shape change through activation of MLCK and inhibition of MLCP, respectively.
Materials and methods
Materials
These studies were approved by the Hull York Medical School Ethics Committee and were conducted in accordance with the Declaration of Helsinki. c-Jun N-terminal kinase (JNK) inhibitor 1, pyrolopyrimidine 2 and 3 (PP2, PP3), Y27632, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM), and ML-7 were obtained from Calbiochem (Nottingham, United Kingdom). R406 was obtained from Selleckchem (Suffolk, United Kingdom). Sulfosuccinimidyl oleate (SSO), antibodies to CD36 (FA6.152), Syk, and phospholipase C γ2 (PLCγ2) were obtained from Santa Cruz (Wembley, United Kingdom). Phospho-MLCSer19 antibody, phospho-MYPT (myosin phosphatase targeting subunit)1Thr853 antibody, and phosphoSrc-tyr416 antibodies came from Cell Signaling (Hitchen, United Kingdom). Antibodies to β-tubulin, phosphotyrosine (4G10), and IgG control came from Upstate (Watford, United Kingdom). Collagen was obtained from Axis Shield (Dundee, United Kingdom). The RhoA pull-down assay was obtained from Cytoskeleton (Cambridge, United Kingdom). All other chemicals came from Sigma (Poole, United Kingdom).
Platelet preparation
Human blood was taken from drug-free volunteers by clean venepuncture using acid citrate dextrose (29.9 mM of sodium citrate, 113.8 mM of glucose, 72.6 mM of sodium chloride, and 2.9 mM of citric acid (pH 6.4) as anticoagulant. Platelet-rich plasma was obtained by centrifugation of whole blood at 200 g at 20°C for 20 minutes. Platelet-rich plasma was treated with citric acid (0.3 mM) and indomethacin (10 µM) and was centrifuged at 800 g for 12 minutes. The platelet pellet was then suspended in wash buffer (36 mM of citric acid, 10 mM of EDTA, 5 mM of glucose, 5 mM of KCl, and 9 mM of NaCl) and was spun once more. Platelets were finally resuspended at a concentration of 2.5 × 108platelets/mL in modified Tyrodes buffer (150 mM of NaCl, 5 mM of HEPES, 0.55 mM of NaH2PO4, 7 mM of NaHCO3, 2.7 mM of KCl, 0.5 mM of MgCl2, and 5.6 mM of glucose) unless otherwise stated.
Platelet aggregation and shape change
Washed platelets (2.5 × 108 platelets/mL) were treated with oxLDL or native LDL (nLDL; 10-200 μg/mL), and aggregation was recorded under constant stirring conditions (1000 rpm) for 4 minutes using a Chronolog aggregation module-dual channel light aggregometer. To monitor shape change, platelets were preincubated with apyrase (2 U/mL), indomethacin (10 μM), and EGTA (1 mM), before addition of LDL.
Platelet-spreading analysis by microscopic analysis
Glass microscope slides were coated with nLDL or oxLDL (50 µg/mL) for 12 hours at 4°C. Washed platelets (5 × 107 platelets/mL) were adhered for 30 minutes at 37°C in the presence of tirofiban (2 μM).21 In some cases, platelets were incubated with the CD36-blocking agent SSO (75 µM), FA6.152 (1 μg/mL), IgG (1 μg/mL), or PP2 or PP3 (20 μM) for 20 minutes at 37°C before adhesion. Adherent platelets were stained for 1 hour with isothiocyanate-phalloidin and were viewed with an IX71 fluorescence microscope using a XM10 CCD camera (Olympus, Japan). Images were captured under ×60 magnification and were analyzed with IMAGEJ software (National Institutes of Health, Bethesda, MD).
LDL preparation and oxidation
LDL was prepared from fresh human plasma by sequential density ultracentrifugation,22 and protein concentration was determined by a modified Lowry assay.23 LDL was oxidized in the presence of CuSO4 (10 µM) at 37°C for 24 hours, before being dialyzed against phosphate buffer (140 mM of NaCl, 8.1 mM of Na2HPO4, 1.9 mM of NaH2PO4, pH 7.4) containing EDTA (100 µM) extensively to remove copper ions.24 The extent of oxidation was determined by measurement of lipid hydroperoxides (LPOs) and relative electrophoretic mobility on agarose gels. For measurement of LPOs, LDLs (100 µg/mL) were mixed with color reagent (163 mM of KH2PO4, 37 mM of K2HPO4, 120 mM of KI, 2g/L of Triton X-100, 0.15 mM of sodium azide, 0.1g/L of alkylbenzyldimethylammonium chloride, and 0.01 mM of (NH4)2MoO4), incubated in the dark for 60 minutes and absorbance read at 365 nm. LPO levels of nLDL were 9.9 ± 7.2 nmoL/mg protein and of oxLDL were 74.0 ± 11.5 nmoL/mg of protein (P < .05 compared with nLDL).25 Samples of agarose gels (1%) were loaded with 20 µg of both nLDL and oxLDL and were run at 100V for 60 minutes. Gels were then stained with Commassie blue stain, and relative electrophoretic mobility was calculated for nLDL (1) and oxLDL (3.9 ± 0.2; P < .05 compared with nLDL).
Immunoprecipitation and immunoblotting
For signaling studies, suspended platelets (3-5 × 108 platelets/mL) were incubated with apyrase (2 U/mL), indomethacin (10 μM), and EGTA (1 mM) to prevent secondary signaling events. Platelets were then treated with nLDL or oxLDL (0-200 μg/mL; for 0.25-5 minutes) before termination with Laemmli buffer. In some cases, platelets were incubated with SSO (50 µM), CD36-blocking antibody FA6.152 (1 μg/mL) or IgG control for 15 minutes, or ML-7 (5 µM), BAPTA-AM (20 μM), Y27632 (10 μM), PP2 (20 μM), PP3 (20 μM), R406 (1 µM), fucoidan (5 µg/mL) or JNK inhibitor 1 (10 µM) for 20 minutes before addition of oxLDL. For immunoprecipitated proteins, washed platelets (7 × 108 platelets/mL) were stimulated with nLDL or oxLDL for 15 seconds in the presence or absence of the stated inhibitors. Platelets were lysed with ice-cold lysis buffer.26 Syk or PLCγ2 were then immunoprecipitated as described previously.26 Proteins were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to polyvinylidene difluoride membranes. Membranes were blocked for 60 minutes with 10% bovine serum albumin (or 5% milk for MLCP experiments), dissolved in Tris-buffered-saline-Tween (0.1%), then incubated with anti-phospho-MLCSer19 (1:1000), anti-phosphoSrc-tyr416 (1:1000), anti-phosphoMLCP-thr853 (1:250), anti-phospho-tyr (4G10, 1:1000), anti-Syk (1:1000), anti-PLCγ2 (1:1000), or an anti β-tubulin antibody (1:1000). Immunoblots were processed as described previously.27
RhoA activity assay
Activity of RhoA in platelets was assessed using a guanosine triphosphate (GTP)-RhoA pull-down assay. Briefly, washed platelets (5 × 108 platelets/mL) were incubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) before treatment with nLDL or oxLDL (50 µg/mL) for 15 seconds in the presence or absence of PP2 or PP3 (20 µM). Platelets were lysed with ice-cold lysis buffer and immediately snap frozen in liquid nitrogen. Lysates were thawed and incubated with rhotekin-RBD beads to pull down activated RhoA (GTP-bound). Proteins were separated by SDS-PAGE and were transferred to polyvinylidene difluoride membranes as described above.
Statistical analysis
Results are expressed as means ± SEM and were analyzed using the Student t test. The results were considered significant when P values were < .05.
Results
OxLDL induce platelet shape change and phosphorylation of MLC
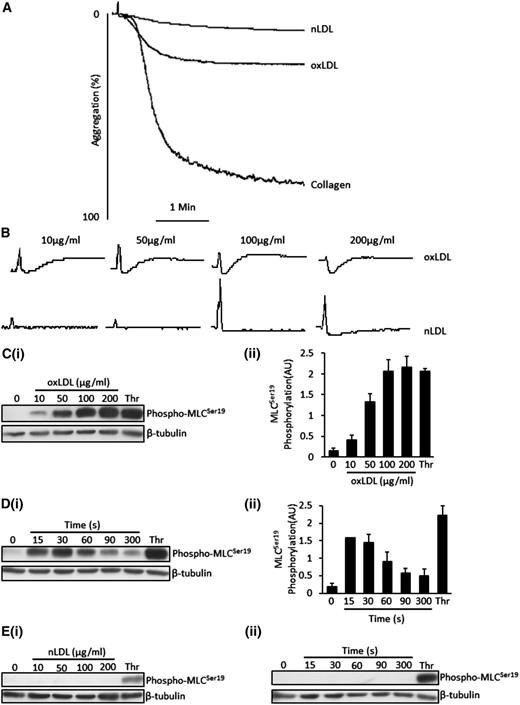
OxLDL, but not nLDL, induced a small degree of aggregation in washed human platelets (20 ± 3% at 50 µg/mL) (Figure 1A). OxLDL-induced aggregation was variable among platelet donors, ranging from 8% to 29% (oxLDL − 50 µg/mL) but consistently caused shape change. The ability of oxLDL to induce shape change was maintained under conditions that inhibited the effects of ADP, TxA2, and integrin signaling, confirming that the effect was independent of other agonists (Figure 1B).
OxLDL induces platelet aggregation, platelet shape change and MLC phosphorylation. (A) Platelets (2.5 × 108/mL) were stimulated with nLDL (50 μg/mL), oxLDL (50 μg/mL), or collagen (10 µg/mL) for 4 minutes under stirring conditions. Shown are representative aggregation traces of 3 separate experiments. (B) Samples were processed as in (A), except that platelets were preincubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with either oxLDL or nLDL (0-200 µg/mL). Traces were recorded for 2 minutes. Shown are representative traces of 3 separate experiments. (C) Platelets (3 × 108/mL) were stimulated with varying concentrations of oxLDL (10-200 µg/mL) or thrombin (0.05 U/mL) for 15 seconds followed by lysis, separation by SDS-PAGE, immunoblot for phospho-MLCSer19, and reprobing for β-tubulin. (Ci) Representative blots. (Cii) Densitometric analysis of 3 independent experiments. (D) Platelets were incubated with 50 µg/mL of oxLDL for various time points (15-300 seconds) or thrombin (0.05 U/mL) for 15 seconds before lysis. Samples were then processed as in (C). (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. (Ei) Samples were processed as in (C), except that platelets were stimulated with nLDL. (Eii) Samples were processed as in (D), except that platelets were stimulated with nLDL. All immunoblots are representatives of 3 separate experiments and were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM). Data are expressed as mean ±SEM.
OxLDL induces platelet aggregation, platelet shape change and MLC phosphorylation. (A) Platelets (2.5 × 108/mL) were stimulated with nLDL (50 μg/mL), oxLDL (50 μg/mL), or collagen (10 µg/mL) for 4 minutes under stirring conditions. Shown are representative aggregation traces of 3 separate experiments. (B) Samples were processed as in (A), except that platelets were preincubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with either oxLDL or nLDL (0-200 µg/mL). Traces were recorded for 2 minutes. Shown are representative traces of 3 separate experiments. (C) Platelets (3 × 108/mL) were stimulated with varying concentrations of oxLDL (10-200 µg/mL) or thrombin (0.05 U/mL) for 15 seconds followed by lysis, separation by SDS-PAGE, immunoblot for phospho-MLCSer19, and reprobing for β-tubulin. (Ci) Representative blots. (Cii) Densitometric analysis of 3 independent experiments. (D) Platelets were incubated with 50 µg/mL of oxLDL for various time points (15-300 seconds) or thrombin (0.05 U/mL) for 15 seconds before lysis. Samples were then processed as in (C). (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. (Ei) Samples were processed as in (C), except that platelets were stimulated with nLDL. (Eii) Samples were processed as in (D), except that platelets were stimulated with nLDL. All immunoblots are representatives of 3 separate experiments and were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM). Data are expressed as mean ±SEM.
A critical event driving platelet shape change is phosphorylation of MLCSer19.28-30 Under the same conditions that prevented secondary signaling, oxLDL (10-200 μg/mL) induced a time- and concentration-dependent increase in phospho-MLCSer19. Increased phosphorylation of MLC was evident at concentrations as low as 10 μg/mL, with maximal effects observed with 100 μg/mL (Figure 1C). The phosphorylation was rapid with 50 μg/mL, inducing maximal effects at 15 seconds before declining back to near-basal levels after 5 minutes (longest time tested) (Figure 1D). In contrast, nLDL failed to stimulate MLC phosphorylation at any of the times or concentrations tested (Figure 1E). Because of the rapid onset of platelet shape change, we used standard conditions of oxLDL (50 μg/mL) for 15 seconds to evaluate the effects of oxLDL on platelet signaling in further experiments.
Platelet shape change and MLC phosphorylation induced by oxLDL require CD36 and occur through both Ca2+-dependent and Rho kinase–dependent pathways
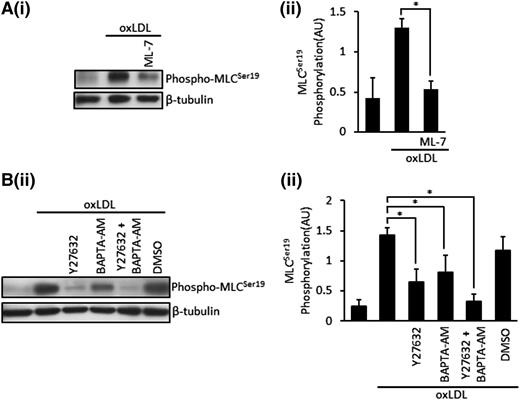
MLC phosphorylation is regulated by a Ca2+ pathway that stimulates MLCK and a Rho kinase (ROCK)–dependent pathway that phosphorylates and inhibits MLCP.28 To further dissect the pathway initiated by oxLDL leading to cytoskeletal reorganization, we evaluated these 2 pathways using ML7 to inhibit MLCK, BAPTA-AM (20 μM) to chelate intracellular Ca2+, and the ROCK inhibitor Y27623 (10 µM). ML-7 (5 µM) significantly reduced, but did not abolish, phospho-MLCSer19 induced by oxLDL (Figure 2A), suggesting that Ca2+-dependent MLCK was responsible for phosphorylation of MLC, but that other pathways could be involved. When BAPTA-AM–treated platelets were stimulated with oxLDL (50 μg/mL), it led to a significant, but incomplete, inhibition of MLC phosphorylation (P < .05) (Figure 2B). Similarly, incubation of platelets with Y27632 before stimulation with oxLDL also significantly reduced MLC phosphorylation (P < .01) without abolishing the response. However, a combination of these inhibitors ablated phospho-MLC (P = .0005) (Figure 2B), suggesting that both Ca2+-dependent and ROCK-dependent pathways were important for oxLDL-induced MLC phosphorylation.
OxLDL induced phosphorylation of MLC are MLCK, Ca2+, Rho kinase dependent. (A) Platelets (3 × 108/mL) were treated with ML-7 (5 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then lysed, separated by SDS-PAGE, and immunoblotted for phospho-MLCSer19 followed by reprobing for β-tubulin. (Ai) Representative blots. (Aii) Densitometric analysis of 5 independent experiments. *P < .05. (B) Platelets (3 × 108/mL) were treated with Y27632 (10 µM), BAPTA-AM (20 µM), or a combination of both or DMSO (0.1%) for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then processed as in (A). (Bi) Representative blots. (Bii) Densitometric analysis of 4 independent experiments. All experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM). Data are expressed as mean ±SEM. *P < .05.
OxLDL induced phosphorylation of MLC are MLCK, Ca2+, Rho kinase dependent. (A) Platelets (3 × 108/mL) were treated with ML-7 (5 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then lysed, separated by SDS-PAGE, and immunoblotted for phospho-MLCSer19 followed by reprobing for β-tubulin. (Ai) Representative blots. (Aii) Densitometric analysis of 5 independent experiments. *P < .05. (B) Platelets (3 × 108/mL) were treated with Y27632 (10 µM), BAPTA-AM (20 µM), or a combination of both or DMSO (0.1%) for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then processed as in (A). (Bi) Representative blots. (Bii) Densitometric analysis of 4 independent experiments. All experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM). Data are expressed as mean ±SEM. *P < .05.
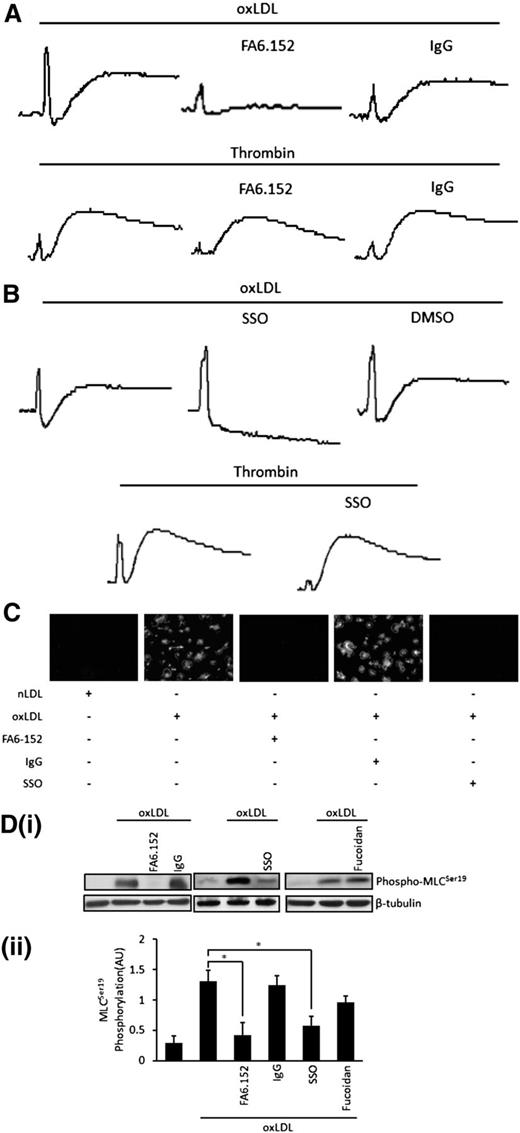
CD36 is highly expressed on platelets31 and has emerged as a potentially key receptor for oxLDL on platelets.9 We explored the possibility that oxLDL-induced shape change and associated signaling responses lay downstream of CD36. This experiment is necessary because oxLDL is proposed to bind to multiple receptors on the platelet surface.6-8 The expression of CD36 on platelets was confirmed by immunoblotting and flow cytometry using specific antibodies (not shown). The role of CD36 was evaluated using the CD36-blocking antibody FA6.152 (1 μg/mL) and the CD36 inhibitor SSO (50 μM).32 Platelet shape change induced by oxLDL (50 µg/mL) in the absence of secondary signaling was abolished by FA6.152 but was unaffected by a control IgG (Figure 3A). Neither of these antibodies influenced shape change initiated by thrombin, which stimulates platelets through Gq and G12/13. Similarly, SSO completely blocked oxLDL-induced shape change but had no effect on that induced by thrombin (Figure 3B).
OxLDL signals through CD36 to induce platelet shape change. (A) Platelets (2.5 × 108/mL) were treated with FA6.152 (1 µg/mL) or control IgG (1 µg/mL) for 15 minutes in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with oxLDL (50 µg/mL) or thrombin (0.05 U/mL). Traces were recorded for 2 minutes. Shown are representative traces of 7 independent experiments. (B) Samples were processed as in (A), except that platelets were treated with SSO (50 μM) or DMSO. Shown are representative traces of 3 separate experiments. (C) Platelets (5 × 107/mL) were adhered to nLDL or oxLDL (50 μg/mL) slides in the presence or absence of SSO (50 μM), FA6.152 (1 µg/mL), or control IgG (1 µg/mL), for 30 minutes and were viewed by fluorescence microscopy. Representative images of 3 independent experiments were taken under ×60 magnification. Bar = 20 µm. (D) Platelets (3 × 108/mL) were treated with FA6.152 (1 µg/mL) or control IgG (1 µg/mL), SSO (50 μM), or fucoidan (5 μg/mL) for 15 minutes in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Platelets were lysed and separated by SDS-PAGE and immunoblotted for phospho-MLCSer19, followed by reprobing for β-tubulin. (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. Data are presented as mean ± SEM. *P < .05.
OxLDL signals through CD36 to induce platelet shape change. (A) Platelets (2.5 × 108/mL) were treated with FA6.152 (1 µg/mL) or control IgG (1 µg/mL) for 15 minutes in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with oxLDL (50 µg/mL) or thrombin (0.05 U/mL). Traces were recorded for 2 minutes. Shown are representative traces of 7 independent experiments. (B) Samples were processed as in (A), except that platelets were treated with SSO (50 μM) or DMSO. Shown are representative traces of 3 separate experiments. (C) Platelets (5 × 107/mL) were adhered to nLDL or oxLDL (50 μg/mL) slides in the presence or absence of SSO (50 μM), FA6.152 (1 µg/mL), or control IgG (1 µg/mL), for 30 minutes and were viewed by fluorescence microscopy. Representative images of 3 independent experiments were taken under ×60 magnification. Bar = 20 µm. (D) Platelets (3 × 108/mL) were treated with FA6.152 (1 µg/mL) or control IgG (1 µg/mL), SSO (50 μM), or fucoidan (5 μg/mL) for 15 minutes in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Platelets were lysed and separated by SDS-PAGE and immunoblotted for phospho-MLCSer19, followed by reprobing for β-tubulin. (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. Data are presented as mean ± SEM. *P < .05.
To further explore the role of CD36 in shape change, we found that adhering platelets to immobilized oxLDL (50 μg/mL), but not nLDL, resulted in spreading. Importantly, blocking of CD36 with either SSO or FA6.152 ablated adhesion and spreading (Figure 3C). Consistent with a role for CD36 in oxLDL-induced shape change and spreading, blocking CD36 with FA6.152 or SSO prevented oxLDL-induced phosphorylation of MLC (P = .02) in platelets (Figure 3D). In contrast, fucoidan, which has been used as an inhibitor of SR-A,8 had only minor effects that were not significant (Figure 3D).
A tyrosine kinase–signaling pathway mediates phosphorylation of MLC in response to oxLDL
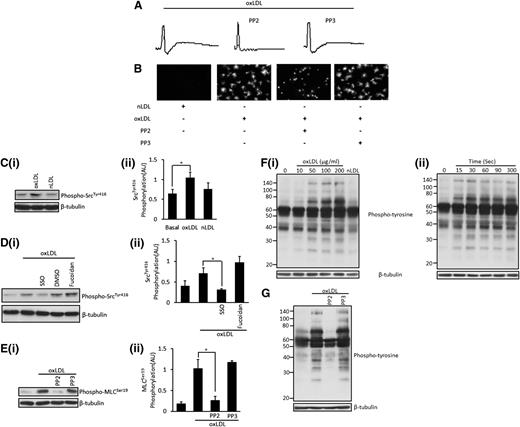
In platelets, Src-family kinases are constitutively associated with CD3633 and lie upstream of signaling pathways diverging from the receptor. We explored their role in CD36-mediated phosphorylation of MLC using a number of approaches. First, we found that shape change of suspended platelets in response to oxLDL was abolished by the presence of the pan Src-family kinase inhibitor PP2 (Figure 4A). In addition, the presence of PP2 abolished platelet spreading on immobilized oxLDL but had no effect on adhesion (Figure 4B). Similar data were observed with the alternative Src-family kinase inhibitor (dasatinib) (supplemental Figure 1). Second, stimulation of platelets with oxLDL, but not nLDL, increased the activatory phosphorylation of Src-family kinases on tyr416 (Figure 4C). Importantly, this phosphorylation event was reduced to basal levels by the CD36 blocker SSO but was unaffected by the SR-A blocker fucoidan (Figure 4D). Third, oxLDL-induced phosphorylation of MLC was completely abolished by PP2 but was maintained in the presence of PP3 (Figure 4E). Together, these observations suggest strongly that Src kinases are critical to the signaling events required for change in platelet shape by oxLDL and that their activation may lie downstream of CD36.
OxLDL induces the activation of Src kinases, which leads to platelet shape change and the phosphorylation of MLC. (A) Platelets (2.5 × 108/mL) were treated with PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL). Traces were recorded for 2 minutes. Shown are representative traces of 6 independent experiments. (B) Platelets (5 × 107/mL) were adhered to nLDL or oxLDL (50 μg/mL) slides in the presence or absence of PP2 or PP3 (20 μM) for 30 minutes and were viewed by fluorescence microscopy. Representative images of 3 independent experiments were taken under ×60 magnification. Bar = 20 µm. (C) Platelets (5 × 108/mL) were stimulated with either oxLDL (50 µg/mL) or nLDL (50 µg/mL) for 15 seconds. Samples were then lysed, separated by SDS-PAGE, and immunoblotted for phospho-SrcTyr416 followed by reprobing for β-tubulin. (Ci) Representative blots. (Cii) Densitometric analysis of 4 independent experiments. *P < .05. (D) Samples were processed as in (C), except that platelets were pretreated with SSO (50 μM) or fucoidan (5 μg/mL). (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. *P < .05. (E) Platelets (3 × 108/mL) were treated with either PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then processed as in (C) and were immunoblotted for phospho-MLCSer19 followed by reprobing for β-tubulin. (Ei) Representative blots. (Eii) Densitometric analysis of 4 independent experiments. *P < .05. (F) Platelets (5 × 108/mL) were stimulated with (Fi) varying concentrations of oxLDL (10-200 µg/mL) or nLDL (50 µg/mL) for 15 seconds. (Fii) Platelets (5 × 108/mL) were incubated with 50 µg/mL of oxLDL for various time points (15-300 seconds) before lysis. Representative blots of 4 independent experiments are shown. (G) Platelets (5 × 108/mL) were treated with either PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples (F-G) were then processed as in (B) and were immunoblotted for phosphotyrosine, followed by reprobing for β-tubulin. All experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM), and are representative of 3 independent experiments.
OxLDL induces the activation of Src kinases, which leads to platelet shape change and the phosphorylation of MLC. (A) Platelets (2.5 × 108/mL) were treated with PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL). Traces were recorded for 2 minutes. Shown are representative traces of 6 independent experiments. (B) Platelets (5 × 107/mL) were adhered to nLDL or oxLDL (50 μg/mL) slides in the presence or absence of PP2 or PP3 (20 μM) for 30 minutes and were viewed by fluorescence microscopy. Representative images of 3 independent experiments were taken under ×60 magnification. Bar = 20 µm. (C) Platelets (5 × 108/mL) were stimulated with either oxLDL (50 µg/mL) or nLDL (50 µg/mL) for 15 seconds. Samples were then lysed, separated by SDS-PAGE, and immunoblotted for phospho-SrcTyr416 followed by reprobing for β-tubulin. (Ci) Representative blots. (Cii) Densitometric analysis of 4 independent experiments. *P < .05. (D) Samples were processed as in (C), except that platelets were pretreated with SSO (50 μM) or fucoidan (5 μg/mL). (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. *P < .05. (E) Platelets (3 × 108/mL) were treated with either PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then processed as in (C) and were immunoblotted for phospho-MLCSer19 followed by reprobing for β-tubulin. (Ei) Representative blots. (Eii) Densitometric analysis of 4 independent experiments. *P < .05. (F) Platelets (5 × 108/mL) were stimulated with (Fi) varying concentrations of oxLDL (10-200 µg/mL) or nLDL (50 µg/mL) for 15 seconds. (Fii) Platelets (5 × 108/mL) were incubated with 50 µg/mL of oxLDL for various time points (15-300 seconds) before lysis. Representative blots of 4 independent experiments are shown. (G) Platelets (5 × 108/mL) were treated with either PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples (F-G) were then processed as in (B) and were immunoblotted for phosphotyrosine, followed by reprobing for β-tubulin. All experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM), and are representative of 3 independent experiments.
To further investigate the significance of tyrosine kinase–dependent signaling events, we measured tyrosine phosphorylation in whole-cell lysates from oxLDL-stimulated platelets. OxLDL, but not nLDL, caused a concentration-dependent increase in tyrosine phosphorylation of a broad range of proteins, with the most prominent bands observed at 25, 32, 70, 90, and 140 kDa (Figure 4Fi). Onset of tyrosine phosphorylation in response to oxLDL (50 μg/mL) occurred within 15 seconds of stimulation and was maintained for 5 minutes (longest time tested) (Figure 4Fii). All experiments were performed in the presence of EGTA, apyrase, and indomethacin, indicating that oxLDL-mediated tyrosine phosphorylation is independent of αIIbβ3-mediated platelet aggregation and platelet-derived agonists. Notably, tyrosine phosphorylation was inhibited by PP2 (Figure 4G), indicating that the effects are likely downstream of Src kinases.
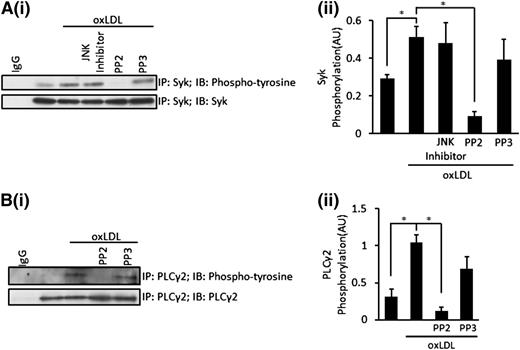
Using immunoprecipitation, we found that oxLDL-stimulated rapid tyrosine phosphorylation of Syk and modest phosphorylation of PLCγ2, which consistent with MLC, was observable after only 15 seconds of stimulation (Figure 5A-B). The central role of Src kinases in the signaling pathway is evidenced by the inhibition of both Syk and PLCγ2 phosphorylation by the presence of PP2, but not PP3 (Figure 5A-B). JNK also lies down of CD36 and Src kinases in platelets,9 but we found that Syk phosphorylation was unaffected by the inhibition of JNK, suggesting that the activation of Syk and PLCγ2 did not require the activation of JNK (Figure 5A). Exploring the role of Syk further, we found that the inhibition of Syk using R40634 partially reduced oxLDL-stimulated phospho-MLC in suspended platelets (Figure 6F) and abolished platelet spreading on oxLDL (supplemental Figure 1).
OxLDL stimulates the activation of Syk and PLCγ2, leading to the phosphorylation of MLC. (A) Platelets (7 × 108) were treated with JNK inhibitor 1 (10 µM), PP2 (20 µM), or PP3 (20 µM) for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Platelets were then lysed and Syk immunoprecipitated. Samples were subsequently separated by SDS-PAGE and were immunoblotted for phosphotyrosine, followed by reprobing for total Syk. (Ai) Representative blots. (Aii) Densitometric analysis of 3 independent experiments. *P < .05. (B) Platelets (7 × 108) were treated with PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Platelets were then lysed and PLCγ2 immunoprecipitated. Samples were subsequently separated by SDS-PAGE and were immunoblotted for phosphotyrosine, followed by reprobing for total PLCγ2. (Bi) Representative blots. (Bii) Densitometric analysis of 3 independent experiments. *P < .05. All experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM). Densitometric data are expressed as mean ±SEM.
OxLDL stimulates the activation of Syk and PLCγ2, leading to the phosphorylation of MLC. (A) Platelets (7 × 108) were treated with JNK inhibitor 1 (10 µM), PP2 (20 µM), or PP3 (20 µM) for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Platelets were then lysed and Syk immunoprecipitated. Samples were subsequently separated by SDS-PAGE and were immunoblotted for phosphotyrosine, followed by reprobing for total Syk. (Ai) Representative blots. (Aii) Densitometric analysis of 3 independent experiments. *P < .05. (B) Platelets (7 × 108) were treated with PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Platelets were then lysed and PLCγ2 immunoprecipitated. Samples were subsequently separated by SDS-PAGE and were immunoblotted for phosphotyrosine, followed by reprobing for total PLCγ2. (Bi) Representative blots. (Bii) Densitometric analysis of 3 independent experiments. *P < .05. All experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM). Densitometric data are expressed as mean ±SEM.
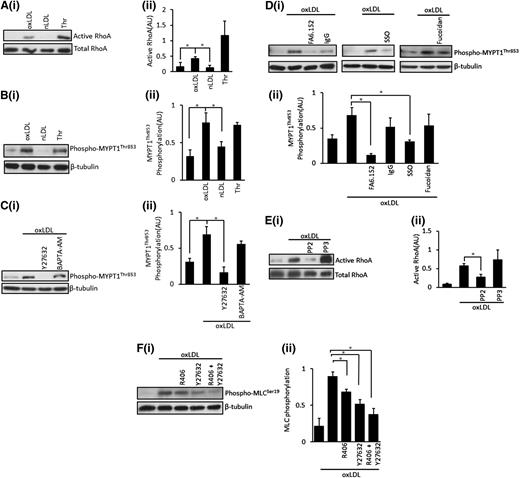
OxLDL, but not nLDL, induces the activation of RhoA and the inhibitory phosphorylation of MLCP. (A) Platelets (5 × 108/mL) were stimulated with oxLDL (50 µg/mL), nLDL (50 µg/mL), or thrombin (0.05 U/mL) for 15 seconds followed by lysis. Samples with then subjected to a RhoA activation assay, separated by SDS-PAGE and immunoblotted for RhoA. (Ai) Representative blots. (Aii) Densitometric analysis of 4 independent experiments. *P < .05. (B) Platelets (5 × 108/mL) were stimulated with oxLDL (50 µg/mL), nLDL (50 µg/mL), or thrombin (0.05 U/mL) for 15 seconds followed by lysis, separation by SDS-PAGE, immunoblot for phospho-MYPT1Thr853, and reprobing for β-tubulin. (Bi) Representative blots. (Bii) Densitometric analysis of 4 independent experiments. *P < .05. (C) Platelets (5 × 108/mL) were treated with Y27632 (10 µM) or BAPTA-AM (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then processed as in (B). (Ci) Representative blots. (Cii) Densitometric analysis of 4 independent experiments. *P < .05. (D) Platelets (5 × 108/mL) were treated with FA6.152 (1 μg/mL), SSO (50 µM), or fucoidan (5 µg/mL) for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds and were processed as in (B). (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. *P < .05. (E) Platelets (5 × 108/mL) were treated with either PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then subjected to a RhoA activation assay and were processed as in (A). (Ei) Representative blots. (Eii) Densitometric analysis of 3 independent experiments. *P < .05. (F) Platelets (3 × 108/mL) were preincubated with Y27632 (10 µM), R406 (1 µM), or a combination of Y27632 and R406 for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds and lysis. Samples were then separated by SDS-PAGE and were immunoblotted for phospho-MLCSer19, followed by reprobing for β-tubulin. (Fi) Representative blots. (Fii) Densitometric analysis of 3 independent experiments. *P < .05. Data are presented as mean ± SEM. Experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM).
OxLDL, but not nLDL, induces the activation of RhoA and the inhibitory phosphorylation of MLCP. (A) Platelets (5 × 108/mL) were stimulated with oxLDL (50 µg/mL), nLDL (50 µg/mL), or thrombin (0.05 U/mL) for 15 seconds followed by lysis. Samples with then subjected to a RhoA activation assay, separated by SDS-PAGE and immunoblotted for RhoA. (Ai) Representative blots. (Aii) Densitometric analysis of 4 independent experiments. *P < .05. (B) Platelets (5 × 108/mL) were stimulated with oxLDL (50 µg/mL), nLDL (50 µg/mL), or thrombin (0.05 U/mL) for 15 seconds followed by lysis, separation by SDS-PAGE, immunoblot for phospho-MYPT1Thr853, and reprobing for β-tubulin. (Bi) Representative blots. (Bii) Densitometric analysis of 4 independent experiments. *P < .05. (C) Platelets (5 × 108/mL) were treated with Y27632 (10 µM) or BAPTA-AM (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then processed as in (B). (Ci) Representative blots. (Cii) Densitometric analysis of 4 independent experiments. *P < .05. (D) Platelets (5 × 108/mL) were treated with FA6.152 (1 μg/mL), SSO (50 µM), or fucoidan (5 µg/mL) for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds and were processed as in (B). (Di) Representative blots. (Dii) Densitometric analysis of 3 independent experiments. *P < .05. (E) Platelets (5 × 108/mL) were treated with either PP2 (20 µM) or PP3 (20 µM) for 20 minutes, followed by stimulation with oxLDL (50 µg/mL) for 15 seconds. Samples were then subjected to a RhoA activation assay and were processed as in (A). (Ei) Representative blots. (Eii) Densitometric analysis of 3 independent experiments. *P < .05. (F) Platelets (3 × 108/mL) were preincubated with Y27632 (10 µM), R406 (1 µM), or a combination of Y27632 and R406 for 20 minutes followed by stimulation with oxLDL (50 µg/mL) for 15 seconds and lysis. Samples were then separated by SDS-PAGE and were immunoblotted for phospho-MLCSer19, followed by reprobing for β-tubulin. (Fi) Representative blots. (Fii) Densitometric analysis of 3 independent experiments. *P < .05. Data are presented as mean ± SEM. Experiments were carried out in the presence of apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM).
OxLDL stimulates a RhoA/ROCK pathway to regulate MLCP
Because MLC phosphorylation in response to oxLDL was prevented in part by the ROCK inhibitor Y27632, we examined the potential regulation of MLCP via RhoA/ROCK. To confirm that oxLDL activated the ROCK-signaling pathways, we examined its upstream activator RhoA. Using a RhoA-GTP pull-down assay, we demonstrate that oxLDL (50 μg/mL) induces activation of RhoA. In contrast, nLDL had no effect on the GTP loading of RhoA (Figure 6A). This finding is consistent with an early study suggesting that modified LDL may activate ROCK, although the mechanisms remained unclear.35 The proposed downstream target of RhoA/ROCK is MLCP, which is dually phosphorylated at thr696/853, with resultant inhibition of phosphatase activity.14,36 OxLDL, but not nLDL, induced inhibitory phosphorylation of thr853 (Figure 6B) and thr696 (not shown). oxLDL-mediated phosphorylation was blocked by Y27632 but was unaffected by chelation of intracellular Ca2+ by BAPTA-AM (Figure 6C). Morerover, oxLDL-stimulated phospho-MYPT1thr853 was ablated by FA6.152 and SSO but not by fucoidan. Together, these data suggest that oxLDL ligation of the scavenger receptor activates ROCK signaling to phosphorylate MYPT1 (Figure 6D). We used the Src kinase inhibitor to explore whether the CD36-Src kinase pathway was responsible for the activation of RhoA. OxLDL induced robust activation of RhoA, which was abolished by PP2 (P = .02) but not by PP3 (Figure 6E). Interestingly, the inhibition of Syk had no effect on the activation of RhoA induced by oxLDL (not shown). Thus, oxLDL stimulates a CD36-Src kinase–dependent activation of RhoA, leading to ROCK activation with resultant phosphorylation and inhibition of MLCP (Figure 6E). Finally, we confirmed that both Src/Syk and ROCK pathways were required for maximal phosphorylation of MLC. Treatment of platelets with the Syk inhibitor R406 and Y27362 alone attenuated, but did not fully block, MLC phosphorylation (Figure 6F). However, when R406 was used in combination with Y27362, the ability of oxLDL to drive the phosphorylation of MLC was abolished (Figure 6F).
Discussion
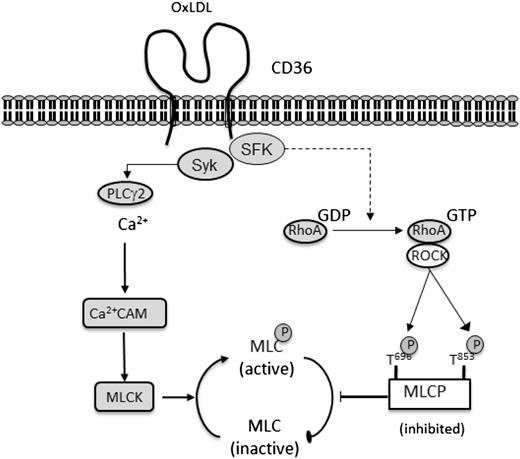
Modified lipoproteins such as oxLDL act as potential pathological ligands that drive platelet activation in disease states. In our present study, we describe new signaling pathways by which oxLDL activates blood platelets. Our data demonstrate for the first time that oxLDL simultaneously activates 2 pathways that lead to the phosphorylation of MLC on Ser19 required for platelet shape change. The ligation of CD36 by oxLDL leads to the sequential activation of Src kinases and Syk, initiating a signaling cascade that leads to tyrosine phosphorylation and activation of PLCγ2. OxLDL-induced signaling events requiring CD36-Src also activates a RhoA/ROCK–dependent pathway that leads to the phosphorylation and inhibition of MLCP. We show that both of these pathways are required for maximal phosphorylation of MLC and platelet activation in response to oxLDL (Figure 7).
OxLDL induces CD36-signaling pathways leading to MLC phosphorylation and change in platelet shape. Diagrammatic representation of the signaling pathways induced by oxLDL, leading to platelet shape change. OxLDL stimulates platelets through CD36 leading to a Src kinase–dependent activation of Syk and PLCγ2, which results in an increase in intracellular Ca2+ levels and activation of MLCK. Simultaneously, a Src kinase–dependent activation of RhoA is initiated, resulting in ROCK activation and inhibition of MLCP inhibition. These events culminate in the phosphorylation of MLC and platelet shape change.
OxLDL induces CD36-signaling pathways leading to MLC phosphorylation and change in platelet shape. Diagrammatic representation of the signaling pathways induced by oxLDL, leading to platelet shape change. OxLDL stimulates platelets through CD36 leading to a Src kinase–dependent activation of Syk and PLCγ2, which results in an increase in intracellular Ca2+ levels and activation of MLCK. Simultaneously, a Src kinase–dependent activation of RhoA is initiated, resulting in ROCK activation and inhibition of MLCP inhibition. These events culminate in the phosphorylation of MLC and platelet shape change.
Focusing on human platelets, our data demonstrate that oxLDL, but not nLDL, induces shape change under conditions that prevent secondary signaling, indicating that the morphologic changes were in direct response to the modified lipoproteins. Consistent with numerous physiological agonists such as thrombin, collagen, and TxA2, oxLDL is able to induce rapid and concentration-dependent phosphorylation of MLC.14,18,28 The ability of oxLDL to induce shape change, spreading, and MLC phosphorylation was abolished in the presence of 2 distinct CD36-blocking agents but not a putative blocker of SR-A. This observation is important because oxLDL can signal through both SR-A and CD36.7,8 Recently, several laboratory studies have suggested that CD36 may be a key receptor for oxLDL that drives both platelet activation and thrombosis,9,11 and in that regard, our data place a central importance of CD36 in the early stages of platelet activation by oxLDL. It is suggested that Src kinases play a key role in transducing signals on ligation of CD36, although the downstream targets remain to be fully established.9,37
Our data provide new insights into the potential roles of CD36-associated Src kinases, which seem to play a critical role in facilitating platelet shape change. Consistent with other studies, we found that oxLDL induced phosphorylation of Src family kinases, which was prevented by blocking CD36. Work by Chen et al suggest that Fyn and Lyn are potential members of the family, which is activated by oxLDL.9 However, phosphorylation of Fyn and Lyn takes up to 15 minutes, but we observed Src family kinase phosphorylation within 15 seconds of exposure. The reason for this finding is unclear, but multiple Src kinases are associated with CD36 in platelets including Fyn, Lyn, and Yes, and it is possible that the kinetics for phosphorylation of these family members are dependent on the conditions. Regardless of which member of the family is responsible, it is clear that platelet shape change, spreading, and MLC phosphorylation seemed to be dependent on Src kinases, suggesting a key role for a tyrosine kinase–dependent signaling pathway. In endothelial cells, Syk has been shown to be associated with CD3638 ; consistent with that observation, oxLDL induced rapid tyrosine phosphorylation of a number of platelet proteins, including Syk and PLCγ2. These events resemble those related to signaling events downstream of GPIb-IX-V and integrin αIIbβ3,39,40 which also lead to activation of Syk. It is possible that activation of Syk and PLCγ2 may drive the Ca2+mobilization required for platelet activation and spreading. Indeed, Nergiz-Unal et al recently demonstrated that immobilized oxLDL can stimulate CD36-dependent mobilization of Ca2+.41 Our work confirms the importance of CD36 and Src but extends these findings significantly to demonstrate that the shape change and spreading are rapid (not requiring extensive interactions with immobilized ligands) and are mediated by phosphorylation of MLC. Unfortunately, we found that our oxLDL did not induce tyrosine phosphorylation in murine platelets (K.S.W. and K.M.N., unpublished data), suggesting that oxLDL may signal differently in human and murine platelets, but also precluding the use of Syk and PLCγ2–deficient murine platelets. However, the central role of Syk is supported by data demonstrating that inhibition of the kinase by R406 reduces phosphorylation of MLC and abolishes platelet spreading induced by oxLDL. Interestingly, inhibition of PLCγ2 using the nonspecific inhibitor U73122 also reduced MLC phosphorylation in response to oxLDL (K.S.W. and K.M.N., unpublished data). This finding could suggest that oxLDL may increase intracellular Ca2+ through activation of PLCγ2, which is required for the activation of MLCK and is consistent with the ability of ML-7, the MLCK inhibitor, to reduce phosphorylation of MLC in response to oxLDL (Figure 7).
A second critical finding of our current study is that, for the first time, we identify RhoA/ROCK as a target of CD36 signaling. The induction of shape change by physiological agonists involves at least 2 pathways: a Ca2+-dependent and a RhoA/ROCK–dependent pathway, with agonists activating platelets via Gq/G12/13 activating both pathways.28 To the best of our knowledge, oxLDL do not signal through Gq/G12/13–coupled receptors. Therefore, our data suggest a novel CD36-signaling target, which may have implications in other cell types. Certainly, the recent observation that RhoA is associated with CD36 in HUVECs supports findings that RhoA activation may occur downstream of CD36 in platelets.38 Using a number of pharmacologic inhibitors to isolate tyrosine kinase, Ca2+, and ROCK pathways, we demonstrate that oxLDL-stimulated MLC phosphorylation was partially independent of Ca2+ because it was still evident under conditions that prevented mobilization of intracellular Ca2+ and signaling through Syk. The remaining MLC phosphorylation under these conditions was prevented by the Rho kinase inhibitor Y27632, suggesting that oxLDL activated dual pathways to drive MLC phosphorylation.
We present 4 pieces of evidence to support activation of a RhoA/ROCK pathway. First, treatment of platelets with oxLDL led to the conversion of RhoA to its GTP-loaded form. Second, the activation of RhoA by oxLDL was prevented by blocking the activity of Src kinases, which lie downstream of CD36. Third, oxLDL induces inhibitory phosphorylation of MLCP-thr696/853 on the MYPT subunit. Fourth, the inhibitory phosphorylation by oxLDL was lost in the absence of both CD36 and ROCK activity, suggesting the existence of a CD36/Src kinase/RhoA/ROCK pathway that leads to phosphorylation and inhibition of MLCP (Figure 7). The mechanism by which Src kinases lead to activation of RhoA is unclear and requires further study.
The identification of multiple signaling pathways emanating from CD36 suggest that its effects are consistent with many other platelet receptors that activate a rich and diverse set of signaling events that drive multiple aspects of platelet function. Src kinases are a critical signaling node downstream of CD36 in platelets, acting as a hub where multiple pathways diverge. Our data demonstrate that both Src/Syk/PLCγ2 and Src/ROCK pathways are required for maximal MLC phosphorylation (Figures 6 and 7). Importantly, our findings show no evident crosstalk between these pathways because inhibition of signaling through Syk and PLCγ2 induced no observable effect on the activation of RhoA (data not shown). The activation of JNK-1 is also linked to the CD36/Src kinase-signaling hub but, again, is divergent from the Syk/PLCγ2 and ROCK pathways because it takes up to 15 minutes to become activated compared with the rapid activation of Syk and RhoA. It is clear that future studies are required to link the multiple signaling events downstream of CD36 to specific aspects of platelet function to enhance our understanding of platelet activation induced by oxLDL and hyperlipidemia.
In conclusion, we have described for the first time that oxLDL induce coordinated targeting of the phosphorylation status of MLC. Our data demonstrate that beyond ligation of CD36 and activation of Src kinases, multiple signaling pathways emerge that lead to the activation of MLCK and inhibition of MLCP. These data further our understanding of the potential mechanism by which oxLDL activate platelets contributing to the unwanted and unregulated platelet activation that occurs in hyperlipidemia and atherothrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.S.W. performed experiments and analyzed and interpreted the data; S.M. and A.A. performed experiments and analyzed the data; Y.W. performed experiments; D.L. provided essential material and contributed to the writing of the manuscript; and K.M.N. designed research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Khalid M. Naseem, Thrombosis Research Laboratory, Laboratory 013/014, Hardy Building, University of Hull, Cottingham Rd, Hull, HU6 7RX United Kingdom; e-mail: khalid.naseem@hyms.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal