Abstract
Smoldering multiple myeloma (SMM) bridges the gap between monoclonal gammopathy of undetermined significance (a mostly premalignant disorder) and active multiple myeloma (MM). Until recently, no interventional study in patients with SMM showed improved overall survival (OS) with therapy as compared with observation. A report from the PETHEMA-GEM (Programa Español de Tratamientos en Hematologica) group described both fewer myeloma-related events and better OS among patients with high-risk SMM who were treated with lenalidomide and dexamethasone. This unique study prompted us to review current knowledge about SMM and address the following questions: (1) Are there patients currently defined as SMM who should be treated routinely? (2) Should the definitions of SMM and MM be reconsidered? (3) Has the time come when not treating is more dangerous than treating? (4) Could unintended medical harm result from overzealous intervention? Our conclusion is that those patients with the highest-risk SMM (extreme bone marrow plasmacytosis, extremely abnormal serum immunoglobulin free light chain ratio, and multiple bone lesions detected only by modern imaging) should be reclassified as active MM so that they can receive MM-appropriate therapy and the paradigm of careful observation for patients with SMM can be preserved.
Introduction
Smoldering multiple myeloma (SMM) was initially recognized in the 1980s.1 It bridged the gap between monoclonal gammopathy of undetermined significance (MGUS; a mostly premalignant disorder) and active multiple myeloma (MM). The classification of SMM is based on levels of circulating monoclonal immunoglobulin, bone marrow plasmacytosis, and end-organ damage. Until the International Myeloma Working Group (IMWG) classification system was developed (Table 1), definitions had varied.2 The minimum and maximum thresholds for immunoglobulin, bone marrow plasmacytosis, presence of bone lesions, and degree of anemia have varied by the study.1,3 The common theme across these studies was the universal recognition that there were asymptomatic patients who exceeded the limits of the definition of MGUS, who could remain without end-organ damage for years, and who outsurvived MM patients with higher tumor burden and/or end-organ damage. Studies performed in the 1980s assessing the role of observation vs early intervention in these patients revealed no prolongation of survival with treatment.4-6 Studies performed over the next 2 decades assessing bisphosphonate and/or thalidomide usage also did not show any clear advantage to instituting therapy in these asymptomatic patients other than fewer skeletal-related events (SREs).7-14 However, a recent report from the PETHEMA-GEM (Programa Español de Tratamientos en Hematologica) group described both fewer calcium increased, renal dysfunction, anemia, and bone lesions (CRAB) events as well as better overall survival (OS) among high-risk SMM patients treated with lenalidomide and dexamethasone.15
Definitions of SMM (asymptomatic)/IMM/evolving MM
| Reference . | n . | % BMPCs, (M protein, g/L), [other criteria] . | Risk factors . | Median TTP and OS . |
|---|---|---|---|---|
| Kyle and Greipp, 19801 | 6 | ≥10, (and ≥ 30), [no anemia, hypercalcemia, or renal insufficiency] | Not available | Selected for no progression at 5 y |
| Alexanian et al, 19883 | 35 | ≥10, (and > 20 but < 45 g/L), [hemoglobin > 10.5 g/dL] | Asymptomatic lytic bone lesions (n = 10), M protein > 30 g/L | TTP all: 19 mo; TTP no bone: 25 mo; OS all: 105 mo; OS no bone: 125 mo |
| IMWG, 20032 | ≥10, (or ≥ 30 g/L), [absence of: high calcium, hemoglobin 2 g/dL below normal or < 10 g/dL, lytic bone lesions or osteoporosis with compression fracture, symptomatic hyperviscosity, amyloidosis, or > 2 bacterial infections/12 mo] | Not available | ||
| DSS IA18,19 | Not stated, (IgG < 50 g/L, IgA < 30 g/L, or urine M component < 4 g/24 h), [hemoglobin > 10 g/dL, calcium < 12 mg/dL, no more than solitary bone lesion] | Not available | OS: 69 mo76 | |
| DSS IIA18,19 | Not stated, (immunoglobulins higher than stage I), [hemoglobin > 8.5 but < 10 g/dL, calcium < 12 mg/dL, more than a solitary bone lesion, but not advanced bone disease] | Not available | OS: 58 mo76 | |
| Alexanian et al, 198016 | 20 | “Indolent MM”: ≥ 15%, (and IgG > 25 g/L or IgA > 10 g/L), [Hb >10.0 g/dL; < 3 lytic bone lesions; no painful compression fracture] | Not available | TTP: 28 mo; OS from IMM: 64 mo; OS from treatment: 36 mo |
| Alexanian et al, 19883 | 16 | Indolent MM ≥ 10 (and ≥ 45 g/dL), [or Hb < 10.5] | Not available | 8 mo |
| Rosinol et al, 200317 | 53 | Evolving MM ≥ 10, (and ≥ 30 g/L or light chain excretion 1 g/24 h), [hemoglobin > 10 g/dL, no bone lesions, renal, or hypercalcemia] | Evolving (n = 22), nonevolving (n = 26); trend toward hemoglobin < 12 g/dL and M protein > 35 g/L were risk factors | TTP nonevolving: 47 mo; TTP evolving: 16 mo; OS from dexamethasone: 98 mo; OS from treatment: 42 mo |
| Cesana et al, 200277 | 127 | 1-y stability required 11%-19%, (or IgG 35-69 g/L, IgA 21-69 g/L, Bence Jones proteinuria 1 g/24 h), [no bone lesions, anemia, hypercalcemia, and renal insufficiency] | BMPC > 10%; IgA M protein; proteinuria | Not given |
| Reference . | n . | % BMPCs, (M protein, g/L), [other criteria] . | Risk factors . | Median TTP and OS . |
|---|---|---|---|---|
| Kyle and Greipp, 19801 | 6 | ≥10, (and ≥ 30), [no anemia, hypercalcemia, or renal insufficiency] | Not available | Selected for no progression at 5 y |
| Alexanian et al, 19883 | 35 | ≥10, (and > 20 but < 45 g/L), [hemoglobin > 10.5 g/dL] | Asymptomatic lytic bone lesions (n = 10), M protein > 30 g/L | TTP all: 19 mo; TTP no bone: 25 mo; OS all: 105 mo; OS no bone: 125 mo |
| IMWG, 20032 | ≥10, (or ≥ 30 g/L), [absence of: high calcium, hemoglobin 2 g/dL below normal or < 10 g/dL, lytic bone lesions or osteoporosis with compression fracture, symptomatic hyperviscosity, amyloidosis, or > 2 bacterial infections/12 mo] | Not available | ||
| DSS IA18,19 | Not stated, (IgG < 50 g/L, IgA < 30 g/L, or urine M component < 4 g/24 h), [hemoglobin > 10 g/dL, calcium < 12 mg/dL, no more than solitary bone lesion] | Not available | OS: 69 mo76 | |
| DSS IIA18,19 | Not stated, (immunoglobulins higher than stage I), [hemoglobin > 8.5 but < 10 g/dL, calcium < 12 mg/dL, more than a solitary bone lesion, but not advanced bone disease] | Not available | OS: 58 mo76 | |
| Alexanian et al, 198016 | 20 | “Indolent MM”: ≥ 15%, (and IgG > 25 g/L or IgA > 10 g/L), [Hb >10.0 g/dL; < 3 lytic bone lesions; no painful compression fracture] | Not available | TTP: 28 mo; OS from IMM: 64 mo; OS from treatment: 36 mo |
| Alexanian et al, 19883 | 16 | Indolent MM ≥ 10 (and ≥ 45 g/dL), [or Hb < 10.5] | Not available | 8 mo |
| Rosinol et al, 200317 | 53 | Evolving MM ≥ 10, (and ≥ 30 g/L or light chain excretion 1 g/24 h), [hemoglobin > 10 g/dL, no bone lesions, renal, or hypercalcemia] | Evolving (n = 22), nonevolving (n = 26); trend toward hemoglobin < 12 g/dL and M protein > 35 g/L were risk factors | TTP nonevolving: 47 mo; TTP evolving: 16 mo; OS from dexamethasone: 98 mo; OS from treatment: 42 mo |
| Cesana et al, 200277 | 127 | 1-y stability required 11%-19%, (or IgG 35-69 g/L, IgA 21-69 g/L, Bence Jones proteinuria 1 g/24 h), [no bone lesions, anemia, hypercalcemia, and renal insufficiency] | BMPC > 10%; IgA M protein; proteinuria | Not given |
This article will try to address the following 3 questions. First, should the definition of SMM be reconsidered such that those patients who are considered to have the highest-risk SMM are added into the active MM category in order to preserve the doctrine that SMM is an entity that can be observed without therapy? Second, has the time come when not treating is more dangerous than treating? Third, alternatively, could unintended medical harm result from overzealous intervention?
Definitions of SMM
Approximately 8% to 20% of patients with MM are recognized by chance without significant symptoms. Kyle and Greipp first used the term “smoldering myeloma” in 1980 (Table 1).1 This expression referred to those patients who satisfied the following criteria: (1) M protein ≥30 g/L, (2) bone marrow plasmacytosis ≥ 10%, (3) no end-organ damage, and (4) no progression of disease at 5 years. At the same time, Alexanian et al explored the use of the term “indolent” myeloma (IMM),16 an entity that allowed for up to 3 lytic bone lesions, a minimum bone marrow plasmacytosis of 15%, and distinct minimum and maximum thresholds for immunoglobulin G (IgG) and IgA. Over time, Alexanian et al also separated the SMM (or asymptomatic MM) from the IMM, assigning a maximum value of M-spike of 45 g/L for the former category.3 Subsequent to the SMM and the IMM classifications came that of evolving MM, which was defined as an M protein that abruptly increases when symptomatic MM develops.17 There was considerable overlap between Durie-Salmon stage (DSS) IA18,19 and SMM. It was not until 2003, when there was international consensus about the definitions of plasma cell disorders, that the criteria for SMM were established.2 Most subsequent publications have used this more uniform definition.
Defining risk in SMM
As shown in Table 2, many investigators have evaluated the risk of progression among patients with SMM. In most of the earlier studies, “high-risk” patients had annual progression rates as high as 25% to 40%, depending on the risk criteria applied, and had survival rates comparable to patients with active myeloma.20 Several of these risk factors, especially those that related to tumor mass, may be useful to identify patients with the most “advanced” disease rather than those with the most aggressive biology.
Prognostic factors for progression of SMM to active MM
| Reference . | n . | % BMPC, (M protein, g/L), [other criteria] . | Risk factors . | Median TTP and OS . |
|---|---|---|---|---|
| Wisloff et al, 199120 | 71 | ≥10, (*or IgA > 15, IgG > 30, Bence Jones proteinuria > 1 g/24 h) | Lytic bone lesions; BMPCs > 20% | TTP 26 mo; OS 45 mo; no risk: TTP 39 mo; either risk: TTP 10 mo |
| Dimopoulos et al, 199324 | 95 | >15, (*and serum M protein < 45 g/L), [any lytic bone lesion was exclusionary; > hemoglobin 10.5 g/dL] | Protein risk: M protein > 30 g/L or proteinuria > 50 mg/24 h; low (n = 27): no factor; intermediate (n = 43): either protein characteristic; high (n = 25): lytic bone lesions and/or both protein risk characteristics | TTP: 26 mo; low: 61 mo; intermediate: 25 mo; high: 10 mo; OS from SMM (from treatment): low: 89 mo (35 mo); intermediate: 92 mo (31 mo); high: 57 mo (41 mo) |
| Witzig et al, 199428 | 57 | >10, (not stated), [no CRAB] | Circulating cells by PBLI (n = 14) | TTP: circulating: 9 mo; no circulating: 30 mo |
| Facon et al, 199522 | 91 | >15%, (*and DSS I) | Hemoglobin < 12 g/dL; BMPC > 20%; M protein > 30 g/L (IgG) or > 25 g/L (IgA); 0 factor (n = 38); 1 factor (n = 35); >1 factor (n = 18) | TTP: 48 mo: 0: > 50 mo; 1: 26 mo; >1 factor: 6 mo; OS from SMM (from treatment): 0: > 70 m (33 mo); 1: 50 mo (31 mo); >1: 38 mo (32 mo) |
| Moulopolous et al, 199533 | 38 | >10, (or M-spike > 25-45 g/L or Bence Jones > 150 mg/d), [hemoglobin > 10.5 g/dL; no lytic bone lesions] | Abnormal MRI | TTP: normal MRI: 43 mo; abnormal MRI: 16 mo; variegated: 22 mo; diffuse: 16 mo; focal: 6 mo |
| Weber et al, 199723 | 101 | See Moupolous et al, 1995 | M protein > 30 g/L; IgA M protein; proteinuria > 50 mg/24 h; low (n = 16): 0; intermediate (n = 65): 1; high (n = 20): 2 or more | TTP: low: 95 mo; intermediate: 39 mo; high: 17 mo; OS from dexamethasone (from treatment): low: 89 mo (26 mo); intermediate: 87 mo (34 mo); high: 51 mo (32 mo) |
| Kyle et al, 200721 | 276 | IMWG | M protein ≥ 30; BMPC ≥ 10%; group A: M protein only (n = 27); group B: BMPC only (n = 143); group C: both (n = 106) | 2-y TTP (5-y TTP): A: 6% (15%); B: 22% (43%); C: 45% (69%) |
| Perez-Persona et al, 200731 | IMWG | 95% aberrant BMPC (absence of CD19 and/or CD45 expression, overexpression of CD56, or weak expression of CD38); immunoparesis of the uninvolved immunoglobulins: neither (n = 28), either (n = 39), both (n = 39) | Median TTP (5-y TTP): neither: NR (4%); either: 73 mo (46%); both: 23 mo (72%) | |
| Dispenzieri et al, 200825 | 273 | IMWG | M protein ≥ 30; BMPC ≥ 10%; involved FLC/uninvolved; FLC ≥ 8; 1 high (n = 81); 2 high (n = 114); 3 high (n = 78) | 2-y TTP (5-y TTP): 1: 12% (25%); 2: 27% (51%); 3: 52% (76%) |
| Hillengass et al, 201034 | 149 | IMWG | Whole-body MRI: low (n = 126): no or 1 focal lesion; high (n = 23): > 1 focal lesion | Median (2-y TTP): low: not reached (20%); high: 13 mo (70%) |
| Rajkumar et al, 201162 | 655 | IMWG | BMPCs ≥ 60% (n = 21)* | Median TTP (2-y TTP): BMPC ≥ 60%: 7 mo (95%) |
| Larsen et al, 201326 | 586 | IMWG | Involved FLC/uninvolved FLC < 100 (n = 496); involved FLC/uninvolved FLC ≥ 100 (n = 90) | Median (2-y TTP; 5-y TTP); low: NR (28%; 53); high: 15 mo (79%; 94%) |
| Bianchi et al, 2013 29 | 91 | IMWG | High: slide based > 5 × 106/L or > 5% PC/100 cIg MNC; low (n = 77); high (n = 14) | Median (2-y TTP): low: 57 mo (24%); high: 12 mo (71%); OS from SMM (from treatment): low: 148 mo (66 mo); high: 49 mo (31 mo) |
| Rago et al, 201359 | 397 | IMWG | Hemoglobin ≤ 12.5; M protein ≥ 2.5; BMPC ≥ 60 (2.5% of patients) | 10-y TTP: 45%; BMPC ≥ 60% had × 5.6 risk of progression |
| Madan et al, 201030 | 175 | IMWG | PCLI < 1%; PCLI ≥ 1% | 2-y TTP (5-y TTP): low: 40% (60%); high: 60% (68%) |
| Rajkumar et al, 201338 | 351 | IMWG | FISH: low: (n = 53), normal or insufficient; standard: (n = 106), t(11;14), maf translocations, other/unknown translocations, or deletion 13/13q; intermediate: (n = 148), trisomies alone; high: (n = 44), t(4;14) or deletion 17p | TTP: low: not reached; standard: 54 mo; intermediate: 34 mo; high: 24 mo; OS from SMM (from treatment): low, 135 mo (60 mo); standard, 147 mo (77 mo); intermediate, 135 mo (86 mo); high risk, 105 mo (60 mo) |
| Kastritis et al, 201327 | 96 | IMWG | Risk factors: involved FLC/uninvolved FLC ≥ 100; BM ≥ 60% | Median TTP: no risk factor: 73 mo; 1 risk factor: 18 mo; both risk factors 8 mo |
| Neben et al, 201239 | 246 | IMWG | High-risk FISHs: t(4:14), deletion 17p or +1q21; high tumor burden: M protein ≥ 20 g/L; FISH and tumor burden: both low risk (n = 81); FISH high risk, tumor low risk (n = 44); FISH low risk, tumor high risk (n = 76); both high risk (n = 44) | 3-y TTP: both low risk: 8%; FISH high risk only: 30%; tumor high risk only: 40%; both high risk: 59% |
| Reference . | n . | % BMPC, (M protein, g/L), [other criteria] . | Risk factors . | Median TTP and OS . |
|---|---|---|---|---|
| Wisloff et al, 199120 | 71 | ≥10, (*or IgA > 15, IgG > 30, Bence Jones proteinuria > 1 g/24 h) | Lytic bone lesions; BMPCs > 20% | TTP 26 mo; OS 45 mo; no risk: TTP 39 mo; either risk: TTP 10 mo |
| Dimopoulos et al, 199324 | 95 | >15, (*and serum M protein < 45 g/L), [any lytic bone lesion was exclusionary; > hemoglobin 10.5 g/dL] | Protein risk: M protein > 30 g/L or proteinuria > 50 mg/24 h; low (n = 27): no factor; intermediate (n = 43): either protein characteristic; high (n = 25): lytic bone lesions and/or both protein risk characteristics | TTP: 26 mo; low: 61 mo; intermediate: 25 mo; high: 10 mo; OS from SMM (from treatment): low: 89 mo (35 mo); intermediate: 92 mo (31 mo); high: 57 mo (41 mo) |
| Witzig et al, 199428 | 57 | >10, (not stated), [no CRAB] | Circulating cells by PBLI (n = 14) | TTP: circulating: 9 mo; no circulating: 30 mo |
| Facon et al, 199522 | 91 | >15%, (*and DSS I) | Hemoglobin < 12 g/dL; BMPC > 20%; M protein > 30 g/L (IgG) or > 25 g/L (IgA); 0 factor (n = 38); 1 factor (n = 35); >1 factor (n = 18) | TTP: 48 mo: 0: > 50 mo; 1: 26 mo; >1 factor: 6 mo; OS from SMM (from treatment): 0: > 70 m (33 mo); 1: 50 mo (31 mo); >1: 38 mo (32 mo) |
| Moulopolous et al, 199533 | 38 | >10, (or M-spike > 25-45 g/L or Bence Jones > 150 mg/d), [hemoglobin > 10.5 g/dL; no lytic bone lesions] | Abnormal MRI | TTP: normal MRI: 43 mo; abnormal MRI: 16 mo; variegated: 22 mo; diffuse: 16 mo; focal: 6 mo |
| Weber et al, 199723 | 101 | See Moupolous et al, 1995 | M protein > 30 g/L; IgA M protein; proteinuria > 50 mg/24 h; low (n = 16): 0; intermediate (n = 65): 1; high (n = 20): 2 or more | TTP: low: 95 mo; intermediate: 39 mo; high: 17 mo; OS from dexamethasone (from treatment): low: 89 mo (26 mo); intermediate: 87 mo (34 mo); high: 51 mo (32 mo) |
| Kyle et al, 200721 | 276 | IMWG | M protein ≥ 30; BMPC ≥ 10%; group A: M protein only (n = 27); group B: BMPC only (n = 143); group C: both (n = 106) | 2-y TTP (5-y TTP): A: 6% (15%); B: 22% (43%); C: 45% (69%) |
| Perez-Persona et al, 200731 | IMWG | 95% aberrant BMPC (absence of CD19 and/or CD45 expression, overexpression of CD56, or weak expression of CD38); immunoparesis of the uninvolved immunoglobulins: neither (n = 28), either (n = 39), both (n = 39) | Median TTP (5-y TTP): neither: NR (4%); either: 73 mo (46%); both: 23 mo (72%) | |
| Dispenzieri et al, 200825 | 273 | IMWG | M protein ≥ 30; BMPC ≥ 10%; involved FLC/uninvolved; FLC ≥ 8; 1 high (n = 81); 2 high (n = 114); 3 high (n = 78) | 2-y TTP (5-y TTP): 1: 12% (25%); 2: 27% (51%); 3: 52% (76%) |
| Hillengass et al, 201034 | 149 | IMWG | Whole-body MRI: low (n = 126): no or 1 focal lesion; high (n = 23): > 1 focal lesion | Median (2-y TTP): low: not reached (20%); high: 13 mo (70%) |
| Rajkumar et al, 201162 | 655 | IMWG | BMPCs ≥ 60% (n = 21)* | Median TTP (2-y TTP): BMPC ≥ 60%: 7 mo (95%) |
| Larsen et al, 201326 | 586 | IMWG | Involved FLC/uninvolved FLC < 100 (n = 496); involved FLC/uninvolved FLC ≥ 100 (n = 90) | Median (2-y TTP; 5-y TTP); low: NR (28%; 53); high: 15 mo (79%; 94%) |
| Bianchi et al, 2013 29 | 91 | IMWG | High: slide based > 5 × 106/L or > 5% PC/100 cIg MNC; low (n = 77); high (n = 14) | Median (2-y TTP): low: 57 mo (24%); high: 12 mo (71%); OS from SMM (from treatment): low: 148 mo (66 mo); high: 49 mo (31 mo) |
| Rago et al, 201359 | 397 | IMWG | Hemoglobin ≤ 12.5; M protein ≥ 2.5; BMPC ≥ 60 (2.5% of patients) | 10-y TTP: 45%; BMPC ≥ 60% had × 5.6 risk of progression |
| Madan et al, 201030 | 175 | IMWG | PCLI < 1%; PCLI ≥ 1% | 2-y TTP (5-y TTP): low: 40% (60%); high: 60% (68%) |
| Rajkumar et al, 201338 | 351 | IMWG | FISH: low: (n = 53), normal or insufficient; standard: (n = 106), t(11;14), maf translocations, other/unknown translocations, or deletion 13/13q; intermediate: (n = 148), trisomies alone; high: (n = 44), t(4;14) or deletion 17p | TTP: low: not reached; standard: 54 mo; intermediate: 34 mo; high: 24 mo; OS from SMM (from treatment): low, 135 mo (60 mo); standard, 147 mo (77 mo); intermediate, 135 mo (86 mo); high risk, 105 mo (60 mo) |
| Kastritis et al, 201327 | 96 | IMWG | Risk factors: involved FLC/uninvolved FLC ≥ 100; BM ≥ 60% | Median TTP: no risk factor: 73 mo; 1 risk factor: 18 mo; both risk factors 8 mo |
| Neben et al, 201239 | 246 | IMWG | High-risk FISHs: t(4:14), deletion 17p or +1q21; high tumor burden: M protein ≥ 20 g/L; FISH and tumor burden: both low risk (n = 81); FISH high risk, tumor low risk (n = 44); FISH low risk, tumor high risk (n = 76); both high risk (n = 44) | 3-y TTP: both low risk: 8%; FISH high risk only: 30%; tumor high risk only: 40%; both high risk: 59% |
cIg, cytoplasmic immunoglobulin; MNC, mononuclear cells; NR, no response; PBLI, peripheral blood labeling index; PC, plasma cells; and PCLI, plasma cell labeling index.
The estimate of bone marrow plasmacytosis was according to the methods of Rajkumar et al35 (ie, using the highest estimate of plasma cells from the aspirate or the bone marrow).
The most commonly identified risk factor for progression to active myeloma in the era before the 2003 IMWG criteria was the number of bone lesions.2,3,18-20 The realization that patients with lytic lesions were among those with the shortest time to requiring systemic therapy contributed to the decision of excluding those patients with lytic bone lesions from the modern SMM definition (Table 2). The size of the M spike and the degree of plasmacytosis were also consistent risk factors (Figure 1A-B).21 The IgA isotype,22,23 the presence of proteinuria,23,24 an abnormal serum immunoglobulin free light chain (FLC) ratio (Figure 1B-C),25-27 circulating plasma cells by slide-based immunofluorescence,28,29 a high proliferative rate of bone marrow plasma cells (BMPCs),30 immunoparesis,31 a high percentage of BMPCs with aberrant flow cytometry (Figure 1D),27,31 and an abnormal magnetic resonance imaging (MRI) (Figure 1E)27,32-34 have also been recognized as risk factors for progression. Bone marrow plasmacytosis as a risk factor is a relatively complex parameter given the variability of estimation, depending on the source of the sample.35 Computed tomography and MRI reveal specific lesions in 40% of DSS I myeloma patients.36 Among asymptomatic MM patients with normal radiographs, 50% have tumor-related abnormalities on MRI of the lower spine.37
Risk of SMM progression to active MM according to different prognostic systems as compared with risk of progression of MGUS to active MM. Gray shading includes 2-year time point. (A) SMM risk based on BMPCs ≥ 10%, M protein ≥ 30 g/L.21 Bold solid line, both above threshold; solid line, BMPCs ≥ 10% but M-protein < 30 g/L; dashed line, BMPC < 10% but M-protein ≥ 30 g/L. (B) SMM risk based on BMPC ≥ 10, M protein ≥ 30 g/L, and involved FLC/uninvolved FLC ≥ 8.25 Bold solid line, all 3 factors above threshold; solid line, any 2 factors above threshold; dashed line, any 1 factor above threshold. (C) SMM risk based on involved FLC/uninvolved FLC ≥ 100.26 Bold solid line, above threshold; solid line, below threshold. (D) SMM risk based on absence of CD19 and/or CD45 expression, overexpression of CD56, or weak expression of CD38 and immunoparesis of either of the uninvolved immunoglobulins.31 Bold solid line, both risk factors present; solid line, either risk factor present; dashed line, neither risk factor present. (E) SMM risk based on presence (bold solid) or absence (solid) of more than 1 focal lesion on whole-body MRI.34 (F) SMM risk based on FISH.38 Bold solid line, del17p,or t(4;14); solid line, trisomies alone; dashed line, any other interphase FISH abnormality; dotted line, normal or insufficient interphase FISH. (G) SMM risk based on high-risk interphase FISH [del17p, t(4;14), +1q21, or hyperdiploidy] and high tumor burden (M-protein ≥ 20 g/L).39 Bold solid line, both high-risk factors present; solid line, interphase FISH low risk and tumor high risk; dashed line, FISH high risk and tumor low risk; dotted line, both low risk. (H) MGUS risk of progression to MM based on M protein ≥ 30 g/L, abnormal rFLC, and heavy chain IgA or IgM.60 Bold solid line, all risk factors present; solid line, 2 risk factors present; dashed line, 1 risk factor present; dotted line, no risk factor present.
Risk of SMM progression to active MM according to different prognostic systems as compared with risk of progression of MGUS to active MM. Gray shading includes 2-year time point. (A) SMM risk based on BMPCs ≥ 10%, M protein ≥ 30 g/L.21 Bold solid line, both above threshold; solid line, BMPCs ≥ 10% but M-protein < 30 g/L; dashed line, BMPC < 10% but M-protein ≥ 30 g/L. (B) SMM risk based on BMPC ≥ 10, M protein ≥ 30 g/L, and involved FLC/uninvolved FLC ≥ 8.25 Bold solid line, all 3 factors above threshold; solid line, any 2 factors above threshold; dashed line, any 1 factor above threshold. (C) SMM risk based on involved FLC/uninvolved FLC ≥ 100.26 Bold solid line, above threshold; solid line, below threshold. (D) SMM risk based on absence of CD19 and/or CD45 expression, overexpression of CD56, or weak expression of CD38 and immunoparesis of either of the uninvolved immunoglobulins.31 Bold solid line, both risk factors present; solid line, either risk factor present; dashed line, neither risk factor present. (E) SMM risk based on presence (bold solid) or absence (solid) of more than 1 focal lesion on whole-body MRI.34 (F) SMM risk based on FISH.38 Bold solid line, del17p,or t(4;14); solid line, trisomies alone; dashed line, any other interphase FISH abnormality; dotted line, normal or insufficient interphase FISH. (G) SMM risk based on high-risk interphase FISH [del17p, t(4;14), +1q21, or hyperdiploidy] and high tumor burden (M-protein ≥ 20 g/L).39 Bold solid line, both high-risk factors present; solid line, interphase FISH low risk and tumor high risk; dashed line, FISH high risk and tumor low risk; dotted line, both low risk. (H) MGUS risk of progression to MM based on M protein ≥ 30 g/L, abnormal rFLC, and heavy chain IgA or IgM.60 Bold solid line, all risk factors present; solid line, 2 risk factors present; dashed line, 1 risk factor present; dotted line, no risk factor present.
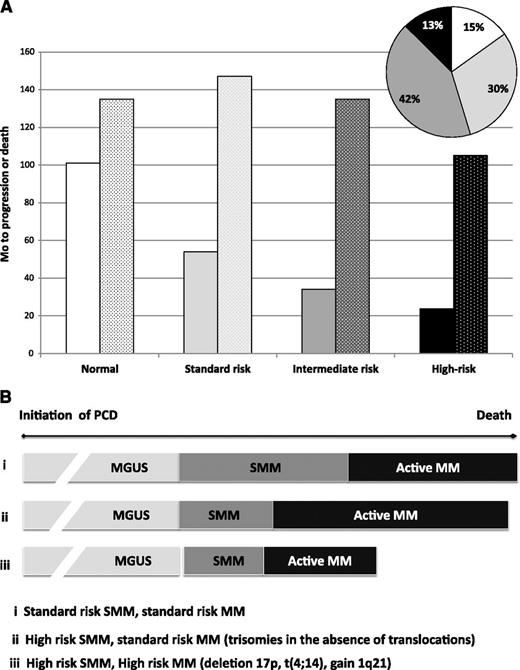
Two groups have recently reported the impact of interphase fluorescence in situ hybridization (FISH) on risk of progression (Table 2 and Figure 1F-G).38,39 Both found that the presence of deletion 17p or t(4;14) is associated with the shortest time to progression (TTP) and that trisomies were a risk factor for progression from SMM to MM. The Mayo paper addressed this peculiar finding of trisomies being a SMM risk factor—but a well-accepted favorable prognostic factor in active MM—by assessing OS in the SMM cohort.38 OS from the time of SMM diagnosis for the trisomy SMM patients was comparable to that of patients with either normal FISH or with standard risk abnormalities such as t(11;14) and deletion 13/13q. This was in stark contrast to outcomes for the deletion 17p and t(4;14) patients, who had inferior OS both from the time of SMM diagnosis and from active MM diagnosis (Figure 2). The Heidelberg group also found that gains of 1q21 were a risk factor for progression among patients with SMM. These authors made an effort to relate FISH abnormalities with other reported risk factors, most notably tumor burden, and found that risk of the high-risk FISH was independent of tumor burden on multivariate analysis, with the greatest impact among those patients with lower tumor burden.39
Distribution and outcomes based on FISH abnormalities among patients with SMM. (A) No interphase FISH abnormalities, white; standard risk: t(11;14), t(14;16), or t(14;20) or other/unknown IgH or del 13/13q, light gray; intermediate risk: trisomy without IgH translocation, dark gray; high risk: t(4;14)or del (17p), black. Solid bars, progression from SMM to MM; stippled bars, OS from SMM diagnosis. (B) Duration of time a patient lives with labels ranging from MGUS to SMM to active MM is in part related to interphase FISH. Although individuals harboring trisomies (ii) appear to progress more rapidly through their diagnosis of SMM than patients with normal FISH or non-t(4;14) translocations (i), they survive much longer than those with deletion 17p (iii) and about as long as patients with normal or non-t(4;14) translocations (i). Mo, months.
Distribution and outcomes based on FISH abnormalities among patients with SMM. (A) No interphase FISH abnormalities, white; standard risk: t(11;14), t(14;16), or t(14;20) or other/unknown IgH or del 13/13q, light gray; intermediate risk: trisomy without IgH translocation, dark gray; high risk: t(4;14)or del (17p), black. Solid bars, progression from SMM to MM; stippled bars, OS from SMM diagnosis. (B) Duration of time a patient lives with labels ranging from MGUS to SMM to active MM is in part related to interphase FISH. Although individuals harboring trisomies (ii) appear to progress more rapidly through their diagnosis of SMM than patients with normal FISH or non-t(4;14) translocations (i), they survive much longer than those with deletion 17p (iii) and about as long as patients with normal or non-t(4;14) translocations (i). Mo, months.
Results of interventional therapeutic trials
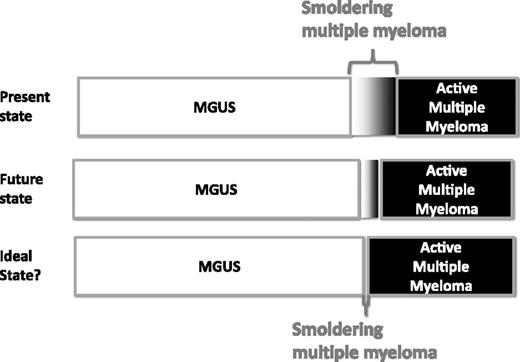
As mentioned, the purpose of the SMM construct was to bridge the gray zone between MGUS and MM (Figure 3). The separation was useful for management because SMM patients had a risk of progression many times greater than MGUS patients and hence needed more frequent follow-up than MGUS patients. Similarly, SMM patients were distinguished from MM because they could be observed without therapy until evidence of disease progression. This strategy was aimed at avoiding unnecessary side effects and cumulative exposure of alkylating drugs, which were found to be associated with myelodysplastic syndrome and acute leukemia.43-46 Patients with DSS I disease, who also meet the criteria for smoldering or asymptomatic myeloma, could be managed expectantly. Median progression-free survival (PFS) in asymptomatic DSS I patients, observed without any therapy, ranged from 12 months to >48 months.4,5,22,47
Present, future, and ideal state for distribution of patients with MGUS, SMM, and MM.
Present, future, and ideal state for distribution of patients with MGUS, SMM, and MM.
Melphalan
Two small randomized clinical trials were reported in the 1990s comparing immediate institution of melphalan and prednisone to initiation only once patients progressed from SMM to symptomatic MM (Table 3). Neither of these trials demonstrated a survival advantage, although they were not adequately powered to make definitive conclusions.4-6
Treatment trials for patients with SMM
| Reference . | Study . | Therapy . | N . | TTP . | OS . |
|---|---|---|---|---|---|
| Hjorth et al, 19934 | RCT | Initial vs delayed MP | 50 SMM and IMM | 12 mo | No difference |
| Riccardi et al, 1994 and 20005,6 | RCT | Initial vs delayed MP | 145 DSS I | ∼12 mo | No difference 64 mo vs 71 mo |
| Peest et al, 199578 | Observational | Delayed MP | 54 DSS I | 2-y PFS 75% | Tumor-specific OS 80% at 60 mo |
| Martin et al, 20027 | Pilot | Pamidronate | 5 SMM and 7 IMM | 2-y TTP 25% | |
| Musto et al, 20038 and D’Arena et al, 20119 | RCT | Pamidronate vs observation | 177 SMM | 5-y PFS 53% both arms; SRE 74% vs 39%, P = .009 | Median OS 46 mo and 48 mo |
| Musto et al, 200810 | RCT | Zoledronate vs observation × 1 y | 163 SMM | TTP: 67 mo vs 59 mo, P = NS SRE: 55% vs 78%, P = .04 | OS not different |
| Barlogie et al, 200811 | Phase 2 | Thalidomide pamidronate | 76 SMM | 4-y EFS 60% | 4-y OS 91% |
| Rajkumar et al, 200112,13 | Phase 2 | Thalidomide | 19 SMM and 10 IMM | Median 35 mo | OS: 86 mo OS from treatment: 49 mo |
| Weber et al, 200314 | Phase 2 | Thalidomide | 28 high-risk SMM | NA | NA |
| Witzig et al, 201348 | RCT | Thalidomide + ZA vs ZA | 68 SMM | 29 mo vs 14 mo | 6 y > 70% |
| Lust et al, 200979 | Ph 2 | Interleukin-1 receptor antagonist ± dexamethasone | 47 SMM and IMM | 37 mo | |
| Golombick et al, 200980 | Crossover | Curcumin vs placebo | 17 SMM | ||
| Mateos et al, 201315 | RCT | Lenalidomide + dexamethasone × 9 mo → lenalidomide maintenance × 15 mo vs observation | 119 SMM | 2-y PFS: 92% vs 50%, P < .001 | 3-y OS: 93% vs 76%, P = .04 |
| Reference . | Study . | Therapy . | N . | TTP . | OS . |
|---|---|---|---|---|---|
| Hjorth et al, 19934 | RCT | Initial vs delayed MP | 50 SMM and IMM | 12 mo | No difference |
| Riccardi et al, 1994 and 20005,6 | RCT | Initial vs delayed MP | 145 DSS I | ∼12 mo | No difference 64 mo vs 71 mo |
| Peest et al, 199578 | Observational | Delayed MP | 54 DSS I | 2-y PFS 75% | Tumor-specific OS 80% at 60 mo |
| Martin et al, 20027 | Pilot | Pamidronate | 5 SMM and 7 IMM | 2-y TTP 25% | |
| Musto et al, 20038 and D’Arena et al, 20119 | RCT | Pamidronate vs observation | 177 SMM | 5-y PFS 53% both arms; SRE 74% vs 39%, P = .009 | Median OS 46 mo and 48 mo |
| Musto et al, 200810 | RCT | Zoledronate vs observation × 1 y | 163 SMM | TTP: 67 mo vs 59 mo, P = NS SRE: 55% vs 78%, P = .04 | OS not different |
| Barlogie et al, 200811 | Phase 2 | Thalidomide pamidronate | 76 SMM | 4-y EFS 60% | 4-y OS 91% |
| Rajkumar et al, 200112,13 | Phase 2 | Thalidomide | 19 SMM and 10 IMM | Median 35 mo | OS: 86 mo OS from treatment: 49 mo |
| Weber et al, 200314 | Phase 2 | Thalidomide | 28 high-risk SMM | NA | NA |
| Witzig et al, 201348 | RCT | Thalidomide + ZA vs ZA | 68 SMM | 29 mo vs 14 mo | 6 y > 70% |
| Lust et al, 200979 | Ph 2 | Interleukin-1 receptor antagonist ± dexamethasone | 47 SMM and IMM | 37 mo | |
| Golombick et al, 200980 | Crossover | Curcumin vs placebo | 17 SMM | ||
| Mateos et al, 201315 | RCT | Lenalidomide + dexamethasone × 9 mo → lenalidomide maintenance × 15 mo vs observation | 119 SMM | 2-y PFS: 92% vs 50%, P < .001 | 3-y OS: 93% vs 76%, P = .04 |
EFS, event-free survival; MP, melphalan-prednisone; NA, not applicable; NS, not significant; RCT, randomized controlled trial; ZA, zoledronic acid.
Bisphosphonates
The next class of drug evaluated in SMM patients in prospective clinical trials (1 small pilot study7 and 2 randomized controlled trials) was single-agent bisphosphonate (Table 3).8-10 Neither of the randomized trials demonstrated improved TTP or OS, but both demonstrated fewer SREs with bisphosphonate use. Patients using bisphosphonate also had higher rates of symptomatic hypocalcemia, fever, and osteonecrosis of the jaw.
Thalidomide
Thalidomide with or without bisphosphonate has been studied in patients with SMM in phase 2 trials and in 1 underpowered randomized controlled trial (Table 3).11-14,48 Eligibility criteria varied among trials as did response rates, PFS, and OS. In the Mayo Clinic randomized controlled trial,48 82% were DSS 1A, but 63% were high risk according to the Mayo Clinic SMM risk classification as defined by Dispenzieri et al.25 There was a significant improvement in PFS in the thalidomide/zoledronic acid arm compared with the zoledronic acid alone arm (29 months vs 14 months) but no difference in PFS as defined by CRAB events (49 months vs 40 months; P = .18) or in OS (6-year OS > 70%).48 The overall response rate was 37% for the thalidomide-containing arm and none with the zoledronic acid group. Thalidomide was poorly tolerated, with 80% of the thalidomide group developing grade 1 or 2 peripheral neuropathy and 74% with grade 1 or 2 fatigue in the thalidomide/zoledronic acid arm. The patients treated with zoledronic acid alone also had adverse effects, including grade 1 or 2 fatigue in 52% and grade 1 or 2 peripheral neuropathy in 18%. The outcomes of this phase 3 trial differed slightly from its phase 2 predecessors in that TTP was shorter than that of Barlogie et al11 or Rajkumar et al12,13 (29 months vs 4-year event-free survival 60% vs 35 months, respectively). Part of the discrepancy may relate to the fact that the Barlogie et al study allowed for all-risk SMM patients and that the Rajkumar et al phase 3 study allowed for patients with IMM to enter. Another discrepancy between these studies is that patients in the Barlogie et al study who achieved a partial response or better had a shorter TTP than the nonresponders, in contrast to the findings of the 2 Mayo-led trials. Indeed, Barlogie et al’s study was concerning in that it implied that treatment with thalidomide may actually select for more aggressive myeloma clones to emerge under the selective pressure of the drug.
Lenalidomide
The most provocative study for patients with SMM is that of the PETHEMA-GEM group.15 These authors reported on 119 patients with high-risk SMM managed in an open-label randomized controlled trial by either observation or lenalidomide and dexamethasone. The lenalidomide and dexamethasone patients received 9 months of induction (28-day cycles of lenalidomide 25 mg/day days 1-21 and dexamethasone 20 mg days 1-4 and 12-15) followed by 15 months of single-agent lenalidomide (10 mg days 1-21 every month). The high-risk population was defined by the presence of both BMPCs >10% and M protein >30 g/L or, if only 1 criterion was present, patients had a proportion of aberrant (defined as absence of CD19 and/or CD45 expression, overexpression of CD56, or weak expression of CD38) plasma cells within the total BMPC compartment by immunophenotyping of ≥95% as well as immunoparesis (reduction under the lower normal limit of either of the uninvolved immunoglobulins).
Patients in the abstention arm were more likely to develop symptomatic disease (76% vs 23%). The overall response rate during induction therapy was 79%, including 65% partial responses, 11% very good partial responses, 14% complete responses, and 7% stringent complete responses. In the treatment group, there were no grade 4 adverse events, but there was 1 death due to pneumonia and 12% of patients had serious adverse events as compared with 3% in the observation arm. Most adverse events were grade 1 or 2. Rates of diarrhea or constipation were 37% (vs 5% in observation arm) and rash occurred in 32%. There were only 3 deep venous thromboses. Seventeen of 57 patients (30%) in the treatment arm withdrew due to toxicity or choice as compared with 3 (4.8%) in the observation arm. A potential impact in quality of life needs to be excluded.
With a median follow-up of 40 months, the treated patients had a superior 3-year survival without progression to symptomatic disease (77% vs 30%; P < .001) and a superior 3-year OS (94% vs 80%; P = .03) from the time of registration. A major limitation in interpreting this study was the difference in how asymptomatic biochemical progression was handled in the 2 groups. In the observation arm, full CRAB progression was required for patients to receive antimyeloma therapy, whereas in the treatment arm, asymptomatic biochemical progression (> 25% increase of monoclonal component) during maintenance lenalidomide was sufficient to warrant salvage with dexamethasone (or reescalation of lenalidomide). A total of 42% (24/57) of patients in the intervention group, who developed asymptomatic biochemical progression, were not counted as events in the Kaplan-Meier curves; rather, 18 had dexamethasone added, and an unspecified fraction had their lenalidomide dose reescalated according to protocol. Finally, the cause for the large discrepancy of discontinuation due to “choice” between the intervention arm vs the abstention arm (23% vs 5%) could be attributed at least in part to patients’ or treating physicians’ reluctance to tolerate asymptomatic biochemical progression in patients for whom therapy had already begun (ie, lenalidomide and dexamethasone). These questions and the design of the study do not allow us to clearly determine if a preemptive strategy may be equally beneficial with less toxicity than a prophylactic strategy.
Moreover, this difference in managing asymptomatic biochemical progression events may explain the relatively high 3-year mortality of 20% in the control arm. Historically, this rate of 3-year mortality is seen in the elderly,49-52 but 3-year mortality for patients with newly diagnosed active myeloma who are transplant eligible is closer to 10% to 15%.53-55 Another caveat is that lenalidomide-dexamethasone was not used consistently as salvage for the abstention group upon progression.
Questions that require clarification before this strategy can be adopted even for high-risk patients include (1) Could some of the excess mortality in the observation arm relate to the protocol requirement that CRAB be reached before instituting therapy? (2) Was there a difference in follow-up compliance and intensity/frequency of de facto testing between the intervention and observation arms? Providing these clarifications will allow this very important study to shed light on questions that extend beyond its primary and secondary objectives.
Rethinking the definition of SMM and timing of therapy
Some have argued that SMM is not a unique biologic state, but rather a heterogeneous entity comprising some patients with biological premalignancy (MGUS) and some with true malignancy who have yet to declare clinical end-organ damage.56,57 With the advent of multiple novel markers of disease (from MRI to positron emission tomography/computed tomography [PET/CT] to flow phenotype to FISH cytogenetics) and of newer (and presumably safer) antimyeloma therapies, should the definition of SMM be reconsidered? We believe so. Patients who are considered to have the highest-risk SMM should be moved into the active MM category in order to preserve the doctrine that SMM is an entity that can be observed without therapy (Figure 3). The time has come when not treating a subset of what has up until now been considered high-risk SMM is more dangerous than treating. Our group has previously shown that even among patients with MGUS, the transition to MM can be unexpected and associated with end-organ damage in 40% of patients who do progress.58 In other studies among patients with SMM who are observed until CRAB, the rates of renal failure were 11% to 13% and SREs 58% to 73%.9,20 In yet another study, 32% of the clinical progressions were severe as defined as the need for red blood cell transfusion, dialysis, or treatment of a pathological fracture.59
Also worthy of consideration is the question of whether some of the lowest-risk SMM patients should be shifted into the MGUS category in order to reduce anxiety and intensity of follow-up, because the absence of risk factors predicts not only a longer TTP, but also a superior OS. To date, annual rates of progression in the “low-risk” SMM are reduced from approximately 10% per year to 3% to 5% per year (Figure 1H). Although this is a significant reduction, these rates of progression are still slightly higher than that of high-risk MGUS.60
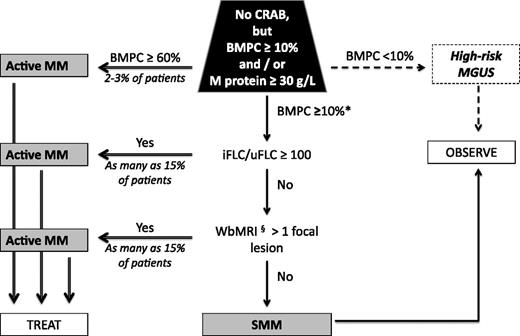
As the questions about treating groups of SMM patients are considered, there must be agreement about acceptable rates of “overtreatment” and “undertreatment” of patients.61 Figure 1 illustrates 2-year progression rates for several recent SMM risk assessments. Most systems contain high-risk groups with 2-year TTP rates of <60% (Table 2). The 4 exceptions are bone marrow plasmacytosis of >60%,27,62 serum immunoglobulin FLC ratio >100 (Figure 1C),26,27 circulating plasma cells by slide-based immunofluorescence,29 and >1 focal lesion on whole-body MRI (Figure 1E).34 Bone marrow plasmacytosis of 60% affects 2% to 8% of all SMM patients, yields a median TTP of 7 months to 15 months,27,62 and had a specificity of 95.5% for progression at 18 months.27 The involved FLC/uninvolved FLC of 100 or greater captures approximately 7% to 15% of the SMM population and had a specificity of 98% for progression at 18 months.27 With a median TTP of 13 to 15 months, a 2-year TTP of 79%, and a 5-year TTP of 94%,26,27 shifting these populations into the active MM category would also be reasonable (Figure 4), though it would be of interest to know how many of these “high-risk” SMM had smoldering light chain myeloma.63 More than 1 focal lesion on whole-body MRI, which affected 15% of SMM patients in one study, had a high predictive value for progression to active MM with a median TTP of 13 months and a 2-year TTP of 70%.34 Diffuse marrow infiltration pattern was also significant on multivariate analysis. Both of these MRI variables made M protein concentration of 40 g/L and bone marrow infiltration of ≥20% insignificant in the multivariate model. Figure 4 summarizes our interpretation of the changing definitions for SMM and active MM.
Algorithm for reclassifying SMM and active MM. *Consider including patients with the following FISH: deletion 17p, t(4;14), and 1q21 gains as active MM; this population could account for as many as 30% of SMM patients. §Consider using more than 1 fluorodeoxyglucose-avid lesion on PET/CT in lieu of MRI. iFLC, involved FLC; uFLC, uninvolved FLC; WbMRI, whole-body MRI.
Algorithm for reclassifying SMM and active MM. *Consider including patients with the following FISH: deletion 17p, t(4;14), and 1q21 gains as active MM; this population could account for as many as 30% of SMM patients. §Consider using more than 1 fluorodeoxyglucose-avid lesion on PET/CT in lieu of MRI. iFLC, involved FLC; uFLC, uninvolved FLC; WbMRI, whole-body MRI.
In terms of other appealing candidates to help redefine SMM and active MM, circulating plasma cells as detected by slide-based immunofluorescence captures 15% of SMM patients and yielded a median TTP of 12 months,29 but this test is not readily available. We therefore await data on a more accessible circulating plasma cell risk system using flow cytometry. Patients with high-risk FISH [deletion 17p, t(4;14), and gain 1q21] might be considered as active myeloma and be candidates for early treatment, but these groups are too heterogeneous for us to make that recommendation.
Consensus recommendations on treatment
Recent work from our institution shows that there has been stage migration64 among those patients being treated as active myeloma, suggesting that patients are being treated at an earlier time point during their disease course. It is possible, but not proven, that some of the improved survival seen in epidemiologic studies65-67 may be in part a function of physicians being more willing to treat patients earlier, which potentially exaggerates the beneficial impact of novel therapies over the past 15 years. The question remains, however, whether treating sooner than later improves quality of life and/or OS. Observation as practiced in the PETHEMA-GEM study in patients with “high-risk” SMM was associated with an unacceptable early mortality that was significantly decreased by early treatment with lenalidomide and dexamethasone. As mentioned earlier, some of the benefit (both survival and time to symptomatic disease) observed in this study may relate to the protocol design: treating biochemical progression and a 30% drop-out rate by “choice” in the treatment arm and strict adherence fulfilling a CRAB criterion prior to instituting therapy in the observation arm.
There are additional caveats that limit the generalizability of the PETHEMA-GEM study. First, the trial results apply not to all patients with SMM, but only to “high-risk” SMM patients as defined by the trial criteria. Forty percent of patients enrolled did so purely based on the flow-based definition of plasma cell immunophenotype, a methodology that is not available in most institutions and that requires considerable expertise to interpret the results even if the technology were available. Second, the authors did not use lenalidomide-dexamethasone as universal salvage for the abstention trial universally. Third, reviewing Figure 1 and Table 2, one sees that this strategy may result in overtreatment of approximately 40% of patients at 3 years, 30% of patients at 4 years, and 20% of patients at 5 years. Fourth, the costs of intervention also need to be considered.61 Although the “cost” of undertreatment is partially captured (more bone lesions and renal failure and now a suggestion of inferior OS), the “cost” of overtreatment is less clear. With the simplest of regimens (ie, lenalidomide plus dexamethasone), the annual cost of therapy is approximately $100 000 USD, not including the extra monitoring required for patients on active therapy and management of adverse events.68,69 Part of the “cost” of overtreatment may include increased side effects, which may translate into inferior quality of life. Finally, long-term safety data for protracted lenalidomide use are limited. The potential of this last “cost” would be abrogated if physicians choose to treat according to the method of the PETHEMA-GEM study (ie, only 2 years of lenalidomide followed by observation until progression), a practice gaining favor in light of concerns of the potential risk of cumulative risk of secondary primary malignancy.70-72
After reviewing all of the data, taking into account the risks and benefits of observation as well as the risks and benefits of intervention, our recommendations for the management of SMM patients are shown in Figure 4. Clearly, there is still room for finding better predictors, but for now we recommend changing the definition of active MM, in the absence of CRAB, to include (1) patients with bone marrow plasmacytosis ≥60%, (2) a ratio of involved to uninvolved FLC of ≥100, or (3) whole-body MRI demonstrating >1 focal lesion. In these patients, the risk of progression in the first 2 or 3 years is 80% or higher. Once defined as having active MM, these patients should receive therapy appropriate for any newly diagnosed patient, and one such therapy now supported with phase 3 evidence would be lenalidomide plus dexamethasone as used in the PETHEMA-GEM interventional arm, though the combination is not Food and Drug Administration approved as first-line therapy in the United States. The cost of performing whole-body MRI on all patients with SMM with the intent of treating only those with >1 focal lesion on MRI would be much less expensive than treating an SMM patient who did not require treatment of 2 years or more. Limitations to using whole-body MRI are that many institutions do not have an algorithm to perform or interpret the test and that payment for the test may not be reimbursed by insurance providers. PET/CT may be a nice alternative to whole-body MRI because PET/CT has a superior sensitivity to standard bone radiographs, is faster and more comfortable for the patient, and can be used in patients with implanted pacemakers and defibrillators.73-75 We recommend that all other patients with SMM be observed without therapy every 3 to 6 months and encouraged to participate in clinical trials. Although the PETHEMA-GEM trial shows that a subset of these patients (those with both BMPCs > 10% and M protein > 30 g/L) could benefit from therapy, we do not recommend intervention at this point until further confirmatory evidence emerges, though it is important that these data be shared with patients. We recommend that our recommendations be considered by the IMWG to arrive at an international consensus.
Acknowledgments
This work was supported in part by the JABBS foundation, the Predolin Foundation, and the Robert A. Kyle Hematologic Malignancies Fund.
Authorship
Contribution: All authors contributed to the design, writing, and review of the manuscript.
Conflict-of-interest disclosure: A.D. received research dollars from Celgene, Millenium, Pfizer, and Janssen. K.S. received Celgene honoraria, Millenium clinical trial funding, and consulting fees from Onyx. R.F. has received a patent for the prognostication of MM based on genetic categorization of the disease, and he has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, Lilly, Onyx, Binding Site, Millennium, and AMGEN; he also has sponsored research from Cylene and Onyx. P.L.B. is an Onyx consultant. M.A.G. received honoraria from Celgene, Millennium, Onyx, and Binding Site. M.Q.L. received research dollars from Celgene. C.R. received research funding from Millennium, Celgene, and Novartis. J.M. received research funding from Celgene, Onyx, and Sanofi. S.K.K. received clinical trial support from Celgene, Millennium, Onyx, Novartis, Cephalon/Teva Oncology, and Abbott and is a consultant (no personal reimbursement) for Millennium, Celgene, and Onyx. The remaining authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.

![Figure 1. Risk of SMM progression to active MM according to different prognostic systems as compared with risk of progression of MGUS to active MM. Gray shading includes 2-year time point. (A) SMM risk based on BMPCs ≥ 10%, M protein ≥ 30 g/L.21 Bold solid line, both above threshold; solid line, BMPCs ≥ 10% but M-protein < 30 g/L; dashed line, BMPC < 10% but M-protein ≥ 30 g/L. (B) SMM risk based on BMPC ≥ 10, M protein ≥ 30 g/L, and involved FLC/uninvolved FLC ≥ 8.25 Bold solid line, all 3 factors above threshold; solid line, any 2 factors above threshold; dashed line, any 1 factor above threshold. (C) SMM risk based on involved FLC/uninvolved FLC ≥ 100.26 Bold solid line, above threshold; solid line, below threshold. (D) SMM risk based on absence of CD19 and/or CD45 expression, overexpression of CD56, or weak expression of CD38 and immunoparesis of either of the uninvolved immunoglobulins.31 Bold solid line, both risk factors present; solid line, either risk factor present; dashed line, neither risk factor present. (E) SMM risk based on presence (bold solid) or absence (solid) of more than 1 focal lesion on whole-body MRI.34 (F) SMM risk based on FISH.38 Bold solid line, del17p,or t(4;14); solid line, trisomies alone; dashed line, any other interphase FISH abnormality; dotted line, normal or insufficient interphase FISH. (G) SMM risk based on high-risk interphase FISH [del17p, t(4;14), +1q21, or hyperdiploidy] and high tumor burden (M-protein ≥ 20 g/L).39 Bold solid line, both high-risk factors present; solid line, interphase FISH low risk and tumor high risk; dashed line, FISH high risk and tumor low risk; dotted line, both low risk. (H) MGUS risk of progression to MM based on M protein ≥ 30 g/L, abnormal rFLC, and heavy chain IgA or IgM.60 Bold solid line, all risk factors present; solid line, 2 risk factors present; dashed line, 1 risk factor present; dotted line, no risk factor present.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/26/10.1182_blood-2013-08-520890/4/m_4172f1.jpeg?Expires=1767705057&Signature=gGb3mJib-RmFKWAyPJca5CIzAkZi3nWT6jIqFn4v9yhP5t6SDJixV3sMCMQ0q6NpoztlY1XiHs0qrh2tOzcGTqgWyP945QR6qDFhbRfLO5P5vdQ93zJebofI4LkcPzWfB5OBom7gHEwbkmpFzY2Uvyc0~BfDnNe1kQnIuKNb1q~TOr4ziemeV9dzid0VBIJBZIkIoL-ga2FNQulwK6JTX-SoWR9vcU48qoQyRsKHpgsUYzrc3WdoUKaRuJYVlTC4WQC0ngbUWoTPLVFX51Oi7Fu4X0lyQJz5uW7WheGwU7QwWafvkAG2sstq5pZDMHHfRqdCBcpS3S8oKCuz25UYUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)