Key Points
Maintenance rituximab attained a prolonged PFS and improved the quality of response in patients with detectable disease after R-FCM.
Abstract
The effectiveness of rituximab maintenance therapy in the treatment of chronic lymphocytic leukemia has been investigated in a phase 2 clinical trial that included an initial treatment with rituximab 500 mg/m2 on day 1 (375 mg/m2 the first cycle), fludarabine 25 mg/m2 on days 1 to 3, cyclophosphamide 200 mg/m2 on days 1 to 3, and mitoxantrone 6 mg/m2 on day 1 (R-FCM), for 6 cycles, followed by a maintenance phase with rituximab 375 mg/m2 every 3 months for 2 years. Sixty-seven patients having achieved complete response (CR) or partial response (PR) with R-FCM were given maintenance therapy. At the end of maintenance, 40.6% of patients were in CR with negative minimal residual disease (MRD), 40.6% were in CR MRD-positive, 4.8% remained in PR, and 14% were considered failures. Six of 29 patients (21%) who were in CR MRD-positive or in PR after R-FCM improved their response upon rituximab maintenance. The 4-year progression-free survival (PFS) and overall survival rates were 74.8% and 93.7%, respectively. MRD status after R-FCM induction was the strongest predictor of PFS. Maintenance with rituximab after R-FCM improved the quality of the response, particularly in patients MRD-positive after initial treatment, and obtained a prolonged PFS. This trial was registered at www.clinicaltrialsregister.eu as identifier #2005-001569-33.
Introduction
Treatment of patients with chronic lymphocytic leukemia (CLL) with chemoimmunotherapy results in high response rates including many cases with no detectable minimal residual disease (MRD). However, all patients eventually relapse and because of this CLL remains an incurable disease.1-6 The lack of sustained responses observed in CLL reflects the persistence of MRD after therapy. Therefore, treatment strategies aimed to eradicate relentless MRD after initial therapy might have a favorable impact on the outcome of patients with CLL.
Throughout the last decade, maintenance treatments mainly based on monoclonal antibodies have been explored in chronic B-cell malignancies, showing benefits in terms of prolongation of progression-free survival (PFS).7-9 In CLL, maintenance or consolidation therapies with different drugs, including interferon-α,10,11 rituximab,12-18 alemtuzumab,19-27 or more recently lenalidomide,28 have been evaluated. As with other B-cell malignancies, the use of rituximab maintenance after induction treatment suggests a benefit in sustaining the response duration in patients with CLL.14
Based on data obtained from in vitro and in vivo studies,29-32 we developed a combination chemotherapy including fludarabine, cyclophosphamide, and mitoxantrone (FCM). The encouraging responses achieved with this combination in patients with CLL, both previously treated and untreated,29,33,34 prompted us to test its combination with rituximab. Therefore, in November 2005, the Spanish Cooperative Group on CLL (GELLC) launched a phase 2 clinical trial aimed at investigating the feasibility, response, and toxicity of a treatment strategy consisting of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) as initial treatment followed by rituximab as maintenance therapy. The initial therapy with R-FCM resulted in an overall response (OR) rate of 93% and a complete response (CR) rate of 82% (46% MRD-negative CR).35 The final results of the second part of the study, namely the maintenance phase with rituximab, are presented here.
Patients and methods
Study design and patients
This open-label prospective, multicenter, nonrandomized phase 2 clinical trial was reviewed and approved by ethical committees in agreement to the Declaration of Helsinki of all centers participating in the study. All patients provided informed written consent.
The trial consisted of 2 parts: an initial treatment with R-FCM followed by maintenance with rituximab. For the initial treatment phase, patients were given R-FCM (rituximab 500 mg/m2 on day 1 [375 mg/m2 the first cycle], fludarabine 25 mg/m2 IV on days 1 to 3, cyclophosphamide 200 mg/m2 on days 1 to 3, and mitoxantrone 6 mg/m2 IV on day 1, given at 4-week intervals) up to a maximum of 6 cycles, as previously published.35 Patients of 70 years or younger presenting active disease according to the National Cancer Institute–sponsored Working Group (NCI-WG) criteria36 and with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 were eligible for the study. Patients with prior history of autoimmune phenomena or a positive Coombs’ test, impaired renal or hepatic function, creatinine clearance inferior of 50 mL per minute, a past history of B or C hepatitis, severe concomitant diseases, or pregnancy were excluded from the study.
The response to initial therapy was assessed 3 months after concluding R-FCM treatment. Patients who obtained a CR or a partial response (PR) were eligible for the maintenance phase of the study. Any severe induction-related event that could impair participation in the maintenance phase precluded eligibility. The main end point of the trial was OR.35 Secondary end points include PFS after maintenance, MRD levels, toxicity treatment, and pharmacokinetic analysis.
Inclusion criteria in this second part of the R-FCM trial were an ECOG performance status of 0 to 2, a neutrophil count superior to 1500/μL, a platelet count superior to 75 000/μL, and CR or PR response to prior R-FCM upfront therapy. Commencing 3 months after the last R-FCM course, patients were scheduled to receive rituximab 375 mg /m2 IV on day 1 and thereafter every 3 months for up to 8 courses (2 years) depending on response and toxicity.
Assessments and response criteria
Patients receiving >4 cycles of maintenance were considered for response evaluation. However, patients in whom rituximab maintenance was prematurely interrupted (≤4 cycles) due to toxicity were considered failures. The response to the maintenance phase was assessed 3 months after the end of treatment using NCI-WG criteria36 and included clinical history, physical examination, white blood cell count (WBC) with differential count, liver and renal function tests, serum lactate dehydrogenase (LDH), and β2-microglobulin and immunoglobulin levels. Bone marrow infiltration was assessed by needle aspiration and biopsy. Patients in CR with no detectable MRD were categorized as MRD-negative CR. Bone marrow biopsy was not required in cases that did not attain clinical CR. Imaging studies were not used to evaluate response to therapy. Patients were assessed by clinical examination every 12 weeks during the 2-year maintenance phase of the study.
Prospective MRD monitoring was a predefined secondary objective of the study. MRD was centrally evaluated using multiparametric flow cytometry assays in paired peripheral blood (PB) and bone marrow (BM) samples 3 months after R-FCM induction therapy, every 6 months during rituximab maintenance, and at the final restaging 3 months after the conclusion of treatment. At completion of the maintenance treatment, MRD was assessed in PB every 3 months during the first year, every 4 months during the second and third year, and every 6 months thereafter and in BM every 6 months during the first year, every 8 months during the second and third year, and every 12 months thereafter. MRD evaluation was performed only in CR MRD-negative patients until MRD became detectable. Whole PB or BM samples were incubated with quadruple combinations of antibodies in a 5-tube combination assay with a sensitivity of 10−4 and analyzed following the method described by Rawstron et al.37 MRD levels are reported as a fraction of CLL cells of all nucleated cells.
Pharmacokinetic analysis
Blood sampling time was scheduled as follows: immediately before the rituximab infusion, and after 4, 12, 24, 48, and 72 hours, and at 7, 14, 28, and 48 days. Rituximab levels were measured with a validated enzyme-linked immunosorbent assay (ELISA) with a quantification limit >0.5 μg/mL. The pharmacokinetic parameters for rituximab were determined performing a 2-compartment open-model analysis with first-order distribution rates between compartments by using the pharmacokinetic software WinNonlin (Version 1.1; Scientific Consulting Inc.). The following pharmacokinetic parameters were considered: total body clearance (Cl), volume of the central compartment (Vc), volume of distribution at steady state (Vss), elimination rate constant (T1/2), elimination rate constant for the α-phase (T1/2α), elimination rate constant for the β-phase (T1/2β), area under the concentration-time curve (AUC), maximum serum concentration (Cmax), minimum serum concentration (Cmin; through serum levels), and mean residence time (MRT).
End points and statistical considerations
The main end point of the trial was OR.35 Secondary end points were PFS after maintenance, MRD levels, and treatment toxicity. PFS was defined by the time from entry into the trial until CLL progression or death from any cause and was calculated in an intention-to-treat basis. Time-to-next treatment (TNT) was defined by time from the end of initial treatment until the initiation of the next therapy. Quantitative MRD results were categorized in the following groups: low (<10−4), intermediate (≥10−4 to <10−2), and high (≥10−2). Low-level MRD was considered as MRD-negative, whereas intermediate- and high-level groups were considered as MRD-positive. The Fisher exact test or the χ2 tests were used to analyze the association between patient characteristics and response and to compare the frequency of adverse events. Actuarial survival curves were estimated by the method of Kaplan and Meier and compared by the log-rank test. Cox regression analysis was performed to analyze the adjusted prognostic value of MRD in a model that also included well-established prognostic factors. All statistical tests were 2-sided and the significance level was 0.05.
Results
Patients’ characteristics and response to therapy
Results of the response obtained with the R-FCM initial treatment in 72 patients were previously published.35 The final results obtained in 81 patients were as follows: CR MRD-negative, 47% (95% CI, 36%-58%); CR MRD-positive, 30% (95% CI, 20%-40%); PR, 13% (95% CI, 6%-20%); and 10% (95% CI, 3%-16%) failed treatment.
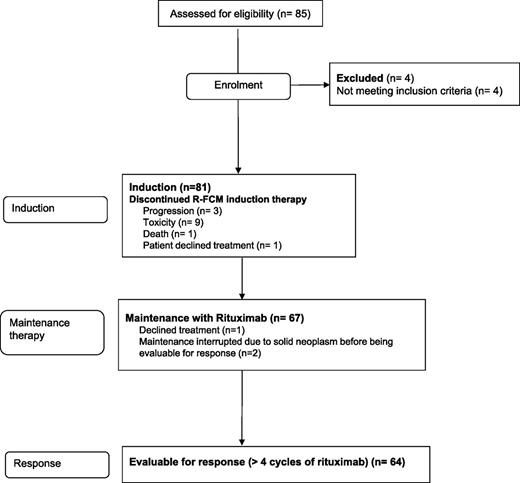
Between July 2006 and November 2008, 67 patients who received upfront treatment with R-FCM were given rituximab maintenance. Two patients were not considered for response evaluation due to the diagnosis of lung cancer after 1 and 2 cycles of rituximab maintenance; an additional patient declined treatment after 2 courses of rituximab (Figure 1). Overall, 64 patients (median age, 60 years; range, 35-70 years) were evaluated for response. The main characteristics of the patients are shown in Table 1. Median number of cycles of maintenance administered was 8 (range, 2-8) and 80% of patients completed the entire planned treatment. In 10 patients, maintenance was discontinued prematurely due to toxicity; in 1 patient, due to development of second malignancies; and in 2 patients, due to disease progression.
Main characteristics of the patients evaluable for response (n = 64) at the entry of the trial
| Variables . | Distribution, n (%) . |
|---|---|
| Age, y | |
| <60 | 32 (50) |
| 60-70 | 32 (50) |
| Gender | |
| Female | 19 (30) |
| Male | 45 (70) |
| Binet stage | |
| A | 9 (14) |
| B | 39 (61) |
| C | 16 (25) |
| Rai Stage | |
| 0 | 3 (5) |
| I-II | 43 (67) |
| III-IV | 18 (28) |
| Lymphocyte count | |
| <100 000/µL | 45 (70) |
| ≥100 000/µL | 19 (30) |
| LDT, n = 55 | |
| >12 mo | 29 (53) |
| ≤12 mo | 26 (47) |
| Serum LDH, n = 61 | |
| Normal | 45 (74) |
| Increased | 16 (26) |
| β2-microglobulin, n = 60 | |
| Normal | 20 (33) |
| Increased | 40 (67) |
| Genetic abnormalities, n = 51 | |
| del(13q) | 12 (23) |
| +12 | 6 (12) |
| del(11q) | 7 (14) |
| del(17p) | 2 (4) |
| ZAP-70 expression, n = 59 | |
| <20% | 24 (41) |
| ≥20% | 35 (59) |
| Response to R-FCM induction | |
| CR MRD− | 35 (55) |
| CR MRD+ | 21 (33) |
| PR | 8 (12) |
| Variables . | Distribution, n (%) . |
|---|---|
| Age, y | |
| <60 | 32 (50) |
| 60-70 | 32 (50) |
| Gender | |
| Female | 19 (30) |
| Male | 45 (70) |
| Binet stage | |
| A | 9 (14) |
| B | 39 (61) |
| C | 16 (25) |
| Rai Stage | |
| 0 | 3 (5) |
| I-II | 43 (67) |
| III-IV | 18 (28) |
| Lymphocyte count | |
| <100 000/µL | 45 (70) |
| ≥100 000/µL | 19 (30) |
| LDT, n = 55 | |
| >12 mo | 29 (53) |
| ≤12 mo | 26 (47) |
| Serum LDH, n = 61 | |
| Normal | 45 (74) |
| Increased | 16 (26) |
| β2-microglobulin, n = 60 | |
| Normal | 20 (33) |
| Increased | 40 (67) |
| Genetic abnormalities, n = 51 | |
| del(13q) | 12 (23) |
| +12 | 6 (12) |
| del(11q) | 7 (14) |
| del(17p) | 2 (4) |
| ZAP-70 expression, n = 59 | |
| <20% | 24 (41) |
| ≥20% | 35 (59) |
| Response to R-FCM induction | |
| CR MRD− | 35 (55) |
| CR MRD+ | 21 (33) |
| PR | 8 (12) |
After rituximab maintenance, 40.6% (n = 26 [95% CI, 29%-53%]) of patients were in MRD-negative CR, 40.6% (n = 26 [95% CI, 29%-53%]) in MRD-positive CR, 4.8% (n = 3 [95% CI, 2%-7%]) remained stable in PR, and 14% (n = 9 [95% CI, 6%-22%]) failed treatment. Failures were due to disease progression (4 patients), severe neutropenia (3 patients), infections (1 patient), and death (1 patient).
Disease status was analyzed before and after rituximab maintenance (Table 2). Among 35 patients in MRD-negative CR after R-FCM, 22 patients (63%) maintained the MRD-negative status at the end of maintenance treatment, 9 patients (25.7%) switched from MRD-negative to MRD-positive, and 4 patients failed treatment, 1 due to disease progression, and 3 due to toxicity.
Response rate according to the induction or maintenance treatment phase
| . | Response to rituximab maintenance . | |||
|---|---|---|---|---|
| . | CR MRD− . | CR MRD+ . | PR . | Failure . |
| Response to R-FCM, N = 64 | ||||
| CR MRD−, n = 35 | 22 | 9 | 0 | 4 |
| CR MRD+, n = 21 | 2 | 15 | 0 | 4 |
| PR, n = 8 | 2 | 2 | 3 | 1 |
| . | Response to rituximab maintenance . | |||
|---|---|---|---|---|
| . | CR MRD− . | CR MRD+ . | PR . | Failure . |
| Response to R-FCM, N = 64 | ||||
| CR MRD−, n = 35 | 22 | 9 | 0 | 4 |
| CR MRD+, n = 21 | 2 | 15 | 0 | 4 |
| PR, n = 8 | 2 | 2 | 3 | 1 |
Median time of conversion from negative to positive MRD was 43 months.
No correlation was observed between different biological or clinical variables, such as age (<60 years vs >60 years), lymphocyte doubling time (LDT; cutoff 12 months), increased ZAP-70, increased serum β2-microglobulin and LDH, cytogenetic abnormalities, or Binet stage, and the achievement of a negative MRD status. However, when values of mean CD20 fluorescence intensity (MFI) obtained before the entry into the study were compared with the response achieved, patients with MRD-negative CR after rituximab maintenance had significantly higher CD20 MFI in comparison with patients with other responses (204 ± 255 vs 99 ± 78, respectively; P = .025). These data suggest that CD20 expression measured by MFI could be a predictor of response to the maintenance with rituximab.
Six of 29 patients (21%) with detectable disease (CR MRD-positive or PR) improved their response category upon rituximab maintenance: 2 patients with MRD-positive CR became MRD-negative, whereas 4 patients in PR obtained a CR after maintenance, 2 of them MRD-negative (Table 2).
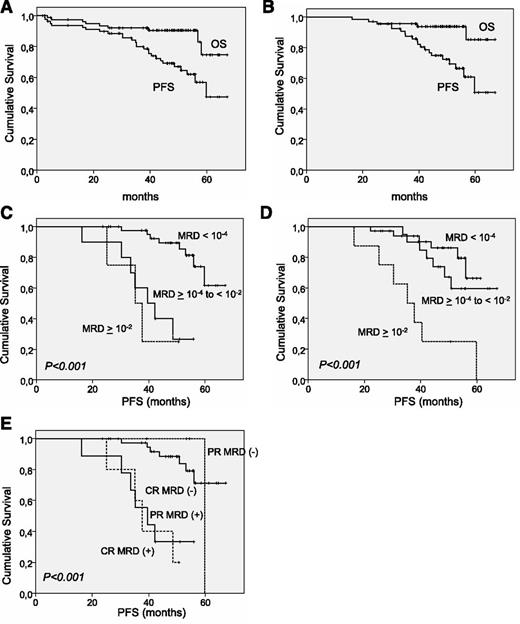
Median follow-up of the whole series (n = 81) was 48.5 months. The 4-year PFS and overall survival (OS) rates were 69.1% (95% CI, 59.3%-78.9%) and 90.5% (95% CI, 78.9%-97.2%), respectively. Median PFS was estimated to be 59.8 months and median OS was not reached (Figure 2). As per those patients who received rituximab maintenance (n = 67), the median follow-up was 48.7 months. The 4-year PFS and OS rates were 74.8% (95% CI, 63.8%-85.8%) and 93.7% (95% CI, 87.6%-99.7%), respectively. Median PFS and OS were not reached (Figure 2).
OS and PFS in the R-FCM trial. (A) OS and PFS in the whole series. (B) OS and PFS in patients entering the rituximab maintenance-study phase. (C) PFS in patients grouped by MRD levels assessed in PB after induction evaluation. (D) PFS according to MRD levels in BM after induction evaluation. (E) PFS combining the degree of response and the MRD status in PB.
OS and PFS in the R-FCM trial. (A) OS and PFS in the whole series. (B) OS and PFS in patients entering the rituximab maintenance-study phase. (C) PFS in patients grouped by MRD levels assessed in PB after induction evaluation. (D) PFS according to MRD levels in BM after induction evaluation. (E) PFS combining the degree of response and the MRD status in PB.
MRD status after R-FCM induction was the strongest predictor of PFS
After initial therapy with R-FCM, MRD was considered to be negative in 76% of patients (45 of 59 patients) in PB and in 55% of patients (35 of 63 patients) in BM. The 4-year PFS rate of patients with negative MRD analyzed in PB was 89.5%, (95% CI, 79%-98%), whereas it was 27% (95% CI, 3%-50%) (P < .01) in patients who had positive MRD in PB. Likewise, patients with MRD-negative in BM had longer PFS in comparison with those with MRD-positive in BM (at 4 years, 86% [95% CI, 73%-99%] vs 60% [95% CI, 42%-78%]; P = .027).
PB and BM paired samples obtained after R-FCM inductions were compared for MRD levels: 12 of 57 patients (21%) with MRD-negative in PB had MRD-positive in BM, whereas all patients with negative MRD in BM also had negative MRD in PB. Patients with MRD-negative in PB but positive in BM (n = 12) presented a similar PFS than those with negative MRD in BM (4-year PFS, 86% [95% CI, 73%-98%] vs 90% [95% CI, 73%-100%], P = .85). Finally, 3 patients who achieved MRD-negative in PB but remained MRD-positive in BM after the initial R-FCM treatment became MRD-negative in BM upon rituximab maintenance.
The impact of different variables, including MRD levels achieved after R-FCM on PFS, was tested in a multivariate analysis. MRD status proved to be a superior predictor for PFS than clinical response (Figure 2). In addition, when different prognostic variables (LDT [cutoff 12 months], ZAP-70, serum β2-microglobulin and LDH, cytogenetic abnormalities, and MRD levels categorized as positive or negative in PB and BM) were analyzed as predictors for PFS, only MRD status in PB along with LDT remained significantly predictive.
According to the criteria used in other trials,38 MRD levels were classified into the following categories: low, <10−4; intermediate, ≥10−4 to <10−2; and high, ≥10−2. When the source of MRD was BM, no significant differences in PFS were observed between patients with negative (n = 35) and intermediate (n = 20) subgroups (at 4 years, 86% [95% CI, 73%-98%] vs 74% [95% CI, 54%-93%], P = .3), whereas patients with high levels of MRD (n = 8) showed an inferior PFS (at 4 years, 25% [95% CI, 0%-55%], P < .01). In contrast, when considering MRD in PB there was no difference in PFS between the intermediate- and high-level MRD groups (at 4 years, 28% [95% CI, 0%-57%] vs 25% [95% CI, 0%-67%], P = .75) (Figure 2).
Toxicity of the maintenance with rituximab
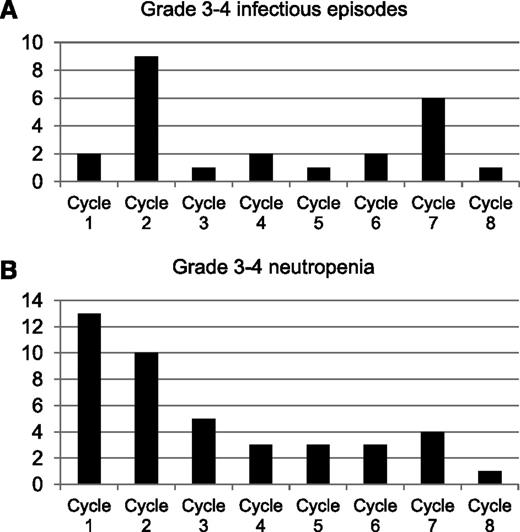
Treatment was delayed due to hematologic toxicity in 9 cycles (2%), 4 of them corresponding to the first cycle, and due to nonhematologic toxicity in 4 cycles (0.8%). No reductions in dose of rituximab were performed. Neutropenia was observed in 31.3% of cycles, although grade 3 to 4 neutropenia was detected only in 8.5% of the cycles (Table 3). Of note, 55% of grade 3 to 4 neutropenia episodes were concentrated in the first 2 cycles of rituximab maintenance (Figure 3). Thrombocytopenia or anemia were infrequent and no grade 3 to 4 toxicities were observed during maintenance with rituximab (Table 3).
Hematologic and extrahematologic toxicity related to rituximab maintenance
| Toxicity . | Total, % . | Grade 1-2, % . | Grade 3-4, % . |
|---|---|---|---|
| Hematologic | |||
| Neutropenia | 31.3 | 22.8 | 8.5 |
| Thrombocytopenia | 4.6 | 4.4 | 0.2 |
| Anemia | 1.2 | 1.2 | 0 |
| Nonhematologic | |||
| Fatigue | 0.8 | 0.8 | 0 |
| Gastrointestinal toxicity | 2 | 2 | 0 |
| Hepatic toxicity | 2.2 | 2 | 0.2 |
| Encephalopathy | 0.2 | 0 | 0.2 |
| Infections | 13 | 8.4 | 4.6 |
| Infectious episodes G 3-4 | |||
| Pneumonia, n = 9 | |||
| Febrile neutropenia, n = 2 | |||
| Cutaneous infection, n = 1 | |||
| Sepsis (Staphylococcus spp), n = 1 | |||
| Cerebral abscess, n = 1 | |||
| Herpes zoster reactivation, n = 1 | |||
| Myositis, n = 1 | |||
| Appendicitis, n = 1 | |||
| Upper respiratory tract, n = 3 | |||
| Progressive multifocal leukoencephalopathy, n = 1 | |||
| Fever of unknown origin, n = 1 | |||
| Neoplasm | |||
| Lung adenocarcinoma, n = 3 | |||
| Epidermoid carcinoma, n = 1 | |||
| Low-grade urothelial carcinoma noninvasive, n = 1 | |||
| Intestinal adenocarcinoma, n = 1 |
| Toxicity . | Total, % . | Grade 1-2, % . | Grade 3-4, % . |
|---|---|---|---|
| Hematologic | |||
| Neutropenia | 31.3 | 22.8 | 8.5 |
| Thrombocytopenia | 4.6 | 4.4 | 0.2 |
| Anemia | 1.2 | 1.2 | 0 |
| Nonhematologic | |||
| Fatigue | 0.8 | 0.8 | 0 |
| Gastrointestinal toxicity | 2 | 2 | 0 |
| Hepatic toxicity | 2.2 | 2 | 0.2 |
| Encephalopathy | 0.2 | 0 | 0.2 |
| Infections | 13 | 8.4 | 4.6 |
| Infectious episodes G 3-4 | |||
| Pneumonia, n = 9 | |||
| Febrile neutropenia, n = 2 | |||
| Cutaneous infection, n = 1 | |||
| Sepsis (Staphylococcus spp), n = 1 | |||
| Cerebral abscess, n = 1 | |||
| Herpes zoster reactivation, n = 1 | |||
| Myositis, n = 1 | |||
| Appendicitis, n = 1 | |||
| Upper respiratory tract, n = 3 | |||
| Progressive multifocal leukoencephalopathy, n = 1 | |||
| Fever of unknown origin, n = 1 | |||
| Neoplasm | |||
| Lung adenocarcinoma, n = 3 | |||
| Epidermoid carcinoma, n = 1 | |||
| Low-grade urothelial carcinoma noninvasive, n = 1 | |||
| Intestinal adenocarcinoma, n = 1 |
Toxicity was based on the NCI-WG and World Health Organization classification and expressed as a percentage of the cycles administered.
Infectious episodes observed in the R-FCM trial during the maintenance phase. (A) Percentage of grade 3 to 4 infectious episodes. (B) Grade 3 to 4 neutropenia episodes by cycle of treatment.
Infectious episodes observed in the R-FCM trial during the maintenance phase. (A) Percentage of grade 3 to 4 infectious episodes. (B) Grade 3 to 4 neutropenia episodes by cycle of treatment.
Sixteen patients experienced grade 3 to 4 infectious episodes, including 9 pneumonia, 2 febrile neutropenia, 1 appendicitis, 1 myositis, 1 cutaneous infection, 1 herpes zoster, 1 sepsis, 3 upper respiratory tract infections, 1 cerebral abscess, and 1 fever of unknown origin. Varicella zoster reactivation occurred in 4 patients. Two patients died, 1 due to due to hemophagocytic syndrome during maintenance and the other due to multifocal leukoencephalopathy 3 months after the end of maintenance therapy. Severe infections (grade 3-4) particularly occurred at the initiation of maintenance treatment, with almost half of all episodes being observed in the first 2 courses of treatment. In addition, a second peak of incidence was observed at the end of maintenance treatment (Figure 3). Infections correlated with the development of severe neutropenia. Thus, grade 3 to 4 infectious episodes appeared in 19.5% of cycles in which grade 3 to 4 neutropenia was observed but in only 3% of cycles with neutropenia inferior to grade 3 (P < .001). Neutropenia of all grades was more frequent in patients with 60 to 70 years (n = 34) than in patients younger than 60 years (n = 33) (39.6% vs 28.6% of cycles, P = .012), although no differences were observed in the number of severe (grade 3-4) infectious events (5.6% vs 3.6% of cycles, P = NS) or grade 3 to 4 neutropenic episodes (10% vs 7.7% of cycles, P = NS) between both age groups. Finally, low-serum immunoglobulin levels of immunoglobulin G (IgG) (<6.5 mg/dL), IgA (<0.7 mg/dL), and IgM (<0.4 mg/dL) were detected in the 58.8%, 49%, and 88.2% of the patients, respectively, at the end of maintenance therapy. Three patients presented levels of IgG lower than 3 mg/dL. At the end of the maintenance therapy, serum immunoglobulin levels were lower than before treatment initiation (supplemental Table 1, available on the Blood Web site). No relationship was observed between infectious events and the presence of low levels of immunoglobulins. T-lymphocyte counts were assessed in PB along with the analysis of MRD. No relationship between low CD4+ T lymphocyte counts (<200/μL) and severe infectious events was observed.
Six nonhematologic neoplasms were observed during the maintenance phase: 3 lung adenocarcinomas, 1 epidermoid carcinoma, 1 low-grade urothelial carcinoma, and 1 intestinal adenocarcinoma. No therapy-related myeloid neoplasms were detected.
Time-to-MRD conversion and TNT is prolonged in R-FCM plus rituximab maintenance as compared with FCM
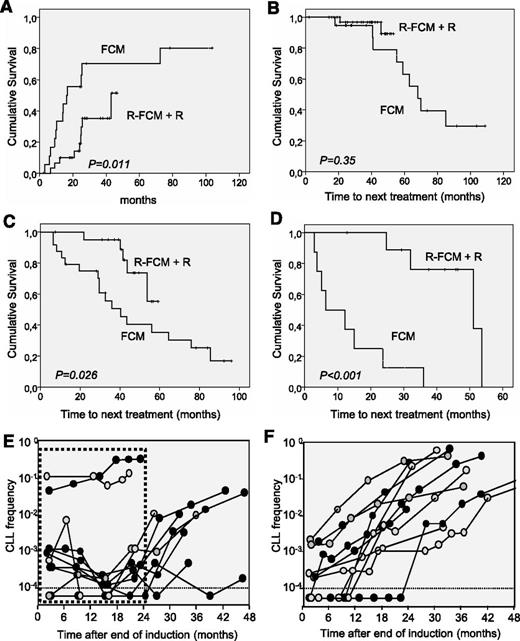
Time-to-MRD conversion and TNT obtained in this trial was compared with that observed in a previous trial from our group using FCM.34 Inclusion criteria were similar between both studies, although the FCM study included patients younger than 65 years, whereas in the R-FCM trial, the upper age limit was 70 years. Patients’ characteristics were not significantly different between the 2 groups, except for a higher percentage of cases with trisomy 12 in the FCM cohort.35 In both studies, MRD was analyzed by FC using a combination of monoclonal antibodies with the same sensitivity. Median time to conversion from MRD-negative to MRD-positive was significantly longer in the R-FCM plus rituximab trial than in the FCM trial, (43 months vs 16.4 months, respectively; P = .011) (Figure 4).
Comparison of the R-FCM with the previous FCM trial.34 (A) Probability of conversion from MRD-negative to MRD-positive in the R-FCM + R and FCM cohorts. (B) TNT in patients who achieved a CR MRD-negative after initial treatment with R-FCM or FCM. (C) TNT in patients who achieved a CR MRD-positive after R-FCM or FCM. (D) TNT in patients who achieved a PR after R-FCM or FCM. (E) MRD kinetics of 11 representative patients of the R-FCM + R cohort. Dotted line box represents period under rituximab maintenance. (F) MRD kinetics of 11 representative patients of the FCM cohort.
Comparison of the R-FCM with the previous FCM trial.34 (A) Probability of conversion from MRD-negative to MRD-positive in the R-FCM + R and FCM cohorts. (B) TNT in patients who achieved a CR MRD-negative after initial treatment with R-FCM or FCM. (C) TNT in patients who achieved a CR MRD-positive after R-FCM or FCM. (D) TNT in patients who achieved a PR after R-FCM or FCM. (E) MRD kinetics of 11 representative patients of the R-FCM + R cohort. Dotted line box represents period under rituximab maintenance. (F) MRD kinetics of 11 representative patients of the FCM cohort.
In addition, compared with the FCM series, TNT in patients attaining a MRD-positive CR (median time of 40.4 months vs nonreached, P = .026) or PR (median time of 6.5 months vs 51 months, P < .001) was significantly prolonged with R-FCM plus maintenance with rituximab. Finally, TNT was not significantly different between R-FCM and FCM in patients obtaining an MRD-negative CR (Figure 4).
To gain insight into the impact of rituximab maintenance on MRD and clinical outcome, kinetics of MRD under rituximab were analyzed. During rituximab maintenance treatment, 72% and 47% of MRD-positive patients show stable or decreased MRD values, even below the limit of detection, in BM and PB, respectively. By contrast, in patients treated with the FCM protocol, once MRD-positive, a steady increase in MRD levels was always observed. Interestingly, once rituximab maintenance was completed in the R-FCM trial, the kinetics of MRD showed a steady increase, mostly resembling the kinetics observed in patients from FCM protocol. In Figure 4E-F, the MRD kinetics of representative patients are depicted.
Rituximab pharmacokinetics
Six patients on maintenance therapy were selected for pharmacokinetic analysis. Five patients obtained a CR and 1 a PR with R-FCM induction. The rituximab levels of all patients on maintenance therapy remained detectable because the levels of all patients on 3-monthly schedule maintenance therapy remained with a mean concentration of 6.2 μg/mL (range, 1.0-11.1 μg/mL). The terminal elimination half-life (T1/2β) of rituximab in all patients was estimated to be 24.6 days (range, 3.4-50.5 days), with an intersubject variability of 64.2%. The mean AUC value was 2675.9 μg × day/L/1.73 m2 (1184.4-3577.4 μg × day/L/1.73 m2) for a normalized dose of rituximab of 375 mg/m2, with an intersubject variability of 35.4%. Vss and Cl were 6981.0 L/1.73 m2 (2960.5-10360.0 L/1.73 m2) and 285.6 mL/day/1.73 m2 (180.0-603.8 mL/day/1.73 m2), respectively. The pharmacokinetic values obtained for each patient are detailed in supplemental Table 2. Of note, the patient in PR had low levels of rituximab in serum in comparison with the 5 patients in CR, which would suggest a possible pharmacokinetic-pharmacodynamic relationship.
Discussion
This study shows that 2 years of rituximab maintenance therapy in patients responsive to first-line combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone results in a prolonged duration of response with a 4-year PFS and OS of 69.1% and 90.5% for the whole series, and 74.8% and 93.7% for patients entering the rituximab maintenance-study phase. These results compare favorably with those observed in the chemoimmunotherapy treatment arm of the German CLL Study Group CLL8 trial (3-year PFS and OS of 65% and 87%),2 whereas they need longer follow-up to be compared with the MD Anderson Cancer Center FCR series.1 The role of rituximab maintenance has been explored with or without prior chemoimmunotherapy.12-18 In line with our results, Del Poeta et al reported that patients receiving consolidation and maintenance with rituximab (n = 28) showed a longer PFS in comparison with a control group (n = 18) not receiving rituximab consolidation (87% vs 32% at 5 years, P = .001).14 Moreover, our results are comparable with those obtained after upfront treatment with FCR-Lite followed by rituximab maintenance administered at higher doses (5-year PFS and OS, 66.9% and 85.5%, respectively).17 Finally, the role of maintenance in CLL is currently explored using other drugs. Thus, the results of consolidation treatment with lenalidomide after upfront therapy with the pentostatin, cyclophosphamide, and rituximab (PCR) combination have been recently reported, showing again a benefit of the maintenance in the treatment of patients with CLL.28
The observation that the interval for conversion from MRD-negative to MRD-positive, and that the TNT was longer in this trial compared with FCM, could be attributable to the effect of rituximab through the control of MRD levels during maintenance. However, this is a retrospective comparison, despite that data from the German CLL Study Group CLL8 trial showing that patients who attained MRD-negative with chemotherapy combinations had a comparable clinical benefit to patients who achieved MRD-negative with chemoimmunotherapy combinations.2,38 To better comprehend the impact of rituximab maintenance on MRD and clinical outcome, the kinetics of MRD under maintenance were analyzed. Rituximab maintenance was able to control MRD levels (negativized, reduced, or maintained levels of residual disease stable) in a proportion of patients. This phenomenon has been reported after allogeneic stem cell transplantation,39 but is not observed after chemotherapy with FCM or autologous stem cell transplantation.40 Once maintenance was finished, levels of MRD steadily increased, mostly resembling the kinetics observed in patients from the FCM protocol.
Finally, variables predicting response duration in other trials, as elevated serum LDH and β2 microglobulin, and high expression of ZAP-70,1,34 did not show adverse impact in the R-FCM plus rituximab maintenance study. In this trial, only MRD levels and LDT predicted PFS duration, parameters that define the quality of response and the kinetics of the disease.
In this study, maintenance therapy was feasible but the hematological and infectious toxicity were not negligible. Previous studies administering rituximab as maintenance treatment in CLL reported inferior hematologic toxicity and a lower number of infectious events than those observed in our study.13-15 This discrepancy may be attributed to differences in intensity of initial treatment regimens used in these trials.13-15 In our study, it is difficult to discriminate which part of the observed toxicity was due to the rituximab maintenance itself or to the initial treatment with R-FCM. In this regard, long-term follow-up of FCR series1,41 disclosed that a substantial number of patients had persistent cytopenia after FCR treatment and a risk of 10% of serious infection was observed during the first year of remission.1,32 In our study, the fact that approximately half of severe neutropenia and infectious episodes were concentrated in the first 2 cycles of maintenance treatment suggests that induction therapy influences the risk of early infections during maintenance. For this reason, treatment strategies including maintenance therapy should take into account prior therapy and toxicity.
In conclusion, R-FCM followed by rituximab maintenance attained a prolonged PFS and improved the quality of response, particularly in patients with detectable disease after upfront R-FCM. Thus, this study supports the concept of the maintenance strategies, either with monoclonal antibodies or other novel therapies, in a disease where all patients eventually relapse. Further prospective, randomized clinical trials are needed to assess the precise role of maintenance strategies in prolonging response and survival in CLL.
Presented in part at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was performed, in part, thanks to grants from the Spanish Ministry of Health, Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias (08/0211 and PI11/00792).
Authorship
Contribution: F.B. and E. Montserrat conceived and designed the study; P.A. provided administrative support; F.B., N.V., M.J.T., E.G.-B., C.F., M.G., E.A., J.D., F.C., J.A.G.-M., L.E., S.F., E. Monzó, Y.G., I.J., and E. Montserrat provided study materials or patients; P.A., F.B., N.V., S.B., X.C., and A.M. collected and assembled data; F.B. and P.A. analyzed and interpreted data; J.B.M. performed pharmacokinetic analysis; F.B., P.A., N.V., J.B.M., and E. Montserrat wrote the manuscript; and P.A., N.V., M.J.T., E.G.-B., M.G., C.F., E.A., J.D., J.A.G.-M., Y.G., F.C., S.F., E. Monzó, I.J., A.M., M.C., L.E., X.C., S.B., J.B.M., E. Montserrat, and F.B. gave final approval of the manuscript.
Conflict-of-interest disclosure: F.B. was a consultant for and received honoraria from Hoffman-La Roche and was a member of an entity’s board of directors or advisory committees. J.D. received lecturing fees from Roche. The remaining authors declare no competing financial interests.
Correspondence: Francesc Bosch, Department of Hematology, University Hospital Vall d'Hebron, Pssg Vall d'Hebron 119-129, 08035, Barcelona, Spain; e-mail: fbosch@vhebron.net.
References
Author notes
E. Montserrat and F.B. contributed equally to this study.