Key Points
Ten cases of an indolent T-cell lymphoproliferative disease of the gastrointestinal tract are reported.
It is important to recognize this condition because it can be mistaken for aggressive T-cell lymphoma, which may lead to unnecessary therapy.
Abstract
Primary gastrointestinal (GI) T-cell lymphoma is an infrequent and aggressive disease. However, rare indolent clonal T-cell proliferations in the GI tract have been described. We report 10 cases of GI involvement by an indolent T-cell lymphoproliferative disease, including 6 men and 4 women with a median age of 48 years (range, 15-77 years). Presenting symptoms included abdominal pain, diarrhea, vomiting, food intolerance, and dyspepsia. The lesions involved oral cavity, esophagus, stomach, small intestine, and colon. The infiltrates were dense, but nondestructive, and composed of small, mature-appearing lymphoid cells. Eight cases were CD4−/CD8+, 1 was CD4+/CD8−, and another was CD4−/CD8−. T-cell receptor-γ chain gene rearrangement identified a clonal population in all 10 cases. There was no evidence of STAT3 SH2 domain mutation or activation. Six patients received chemotherapy because of an initial diagnosis of peripheral T-cell lymphoma, with little or no response, whereas the other 4 were followed without therapy. After a median follow-up of 38 months (range, 9-175 months), 9 patients were alive with persistent disease and 1 was free of disease. We propose the name “indolent T-LPD of the GI tract” for these lesions that can easily be mistaken for intestinal peripheral T-cell lymphoma, and lead to aggressive therapy.
Introduction
Primary T-cell lymphoma of the gastrointestinal (GI) tract and enteropathy-associated T-cell lymphoma (EATL) are rare and aggressive diseases.1-3 Based on the morphology, immunophenotype and genetic characteristics, the World Health Organization classification1 recognizes 2 variants of EATL: types I and II. Type I is often associated with an underlying enteropathy (primarily celiac sprue) and occurs at a higher frequency in northern Europe, where the prevalence of celiac sprue is high. In contrast, type II is usually not associated with enteropathy, occurs most commonly in Asians, and is rare in whites.2,3 Both types of EATL are highly aggressive, recur frequently despite intensive multimodal therapy, and have a poor outcome with a 5-year survival rates of less than 20%.3,4 Several reports in the literature have described clonal T-cell proliferations in the GI tract with an indolent clinical course.5-13 Recently, natural killer (NK) cell enteropathy was recognized as a distinctive benign lymphoproliferative disease mimicking intestinal lymphoma.14,15 These patients do not require aggressive therapy and have a good prognosis.
Herein, we report 10 cases of GI involvement by an indolent T-cell lymphoproliferative disease (T-LPD). One case in this series was the subject of a prior case report.9 We review the clinical, endoscopic, pathologic, and molecular features of this unique cohort, emphasizing the differences from EATL. Some of the patients were initially diagnosed with either inflammatory bowel disease (IBD) or peripheral T-cell lymphoma (PTCL), which led to unnecessary and sometimes aggressive treatments. Furthermore, we propose the name “indolent T-cell lymphoproliferative disease of the GI tract” (or “indolent T-LPD”) for this new pathologic entity to clearly separate it from aggressive PTCL and EATL. Because STAT3 SH2 domain mutations have recently been reported in CD8-positive T-cell large granular lymphocyte leukemia (LGLL),16,17 we performed sequence analysis of this domain and immunostaining for nuclear phospho-STAT3 (pY705-STAT3) in these cases to look for evidence of STAT3 activating mutations or STAT3 activation.
Methods
Immunohistochemistry, in situ hybridization, and clonality studies
Ten cases with available biopsy materials and clinical and laboratory findings were contributed to the study by different coauthors. Six cases were seen in consultation (E.S.J.) by the Hematopathology Section of the National Cancer Institute, Bethesda, MD. All cases were reviewed by A.M.P. and either E.S.J. or W.C.C. to confirm the diagnosis. Formalin-fixed, paraffin-embedded (FFPE) biopsy specimens were sectioned at 4 μm and stained with hematoxylin and eosin. Immunohistochemical stains for CD2, CD3, CD4, CD5, CD7, CD8, CD56, TIA1, granzyme B, T-cell receptor β chain (TCR-BF1), TCR-γ chain (TCR-G), Ki67, and in situ hybridization for Epstein-Barr virus–encoded RNA (EBER) were evaluated against proper internal or external controls. Furthermore, molecular analysis for rearrangement of the TCR-γ chain gene was performed in all cases using the polymerase chain reaction (PCR) on FFPE tissue by the respective contributing institutions according to their established protocols. The study was approved by the Institutional Review Board at the University of Nebraska Medical Center.
Mutation analysis of STAT3
In 5 cases with available tissue, STAT3 mutation analysis was performed. Genomic DNA was isolated using 10 μm FFPE tissue sections using QIAamp DNA FFPE Tissue Kit (Qiagen Inc., Valencia, CA). PCR primers (supplemental Table 1) spanning the mutation hotspots on STAT3 were designed based on 2 recent reports16,17 with IDT primer quest software and optimized with gradient PCR. Fifty to 100 ng of genomic DNA were used as the template for PCR amplification. Specific amplification of the DNA produced PCR amplicons for STAT3-exon-20 and STAT3-exon-21 of 173 bp and 156 bp, respectively, and the amplification was confirmed by Tris-acetate-EDTA–agarose gel electrophoresis. PCR-amplified fragments were purified with the QIAquick PCR purification kit (Qiagen Inc.). The forward and reverse primers used for PCR amplification were also used for Sanger sequencing with Applied Biosystems ABI 3730 48-capillary electrophoresis DNA analyzer (Applied Biosystems, Foster City, CA). The sequencing results were analyzed by aligning the sequences with the National Center for Biotechnology Information STAT3 messenger RNA Reference Sequence (NM_139276) with Vector NTI 10.3.0 software (Invitrogen, Carlsbad, CA).The sequencing chromatograms were visually analyzed with Sequence Scanner v1 software (Applied Biosystems).
Phospho-STAT3 immunohistochemistry
Immunohistochemical stains for pY-STAT3 were performed in parallel with the STAT3 mutation analysis using anti-STAT3 (phospho Y705) antibody (Abcam Inc., Cambridge, MA) at a 1:100 dilution. Antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0 for 20 minutes). Leica Bond Polymer Refine Detection Kit (Leica Microsystems Inc., Buffalo Grove, IL) was used to detect pY-STAT3.
Results
Clinical features
The clinical features of the patients are summarized in Table 1. Among the 10 patients, 6 were male and 4 were female (male/female ratio = 1.5:1) with a median age of 48 years (range, 15-77 year). Data regarding ethnicity were available in 7 patients; 5 were white, 1 African American, and 1 Asian. The most common presenting symptoms were diarrhea (8 patients) associated with abdominal pain or discomfort (6 patients). Other symptoms included vomiting, food intolerance, and dyspepsia. One patient reported drenching night sweats. Another patient (case 9) presented with paresthesias and confusion, and was found to have multiple metabolic abnormalities including hypocalcemia, hypomagnesemia, hypokalemia, and hypozincemia. None of the patients had a history of celiac sprue. Two patients carried a diagnosis of Crohn disease based on prior GI biopsies (not reviewed by the authors). One of these patients (case 5) received multiple treatments including 5-aminosalicylic acid, infliximab, 6-mercaptopurine, mycophenolate mofetil, methotrexate, and anti–tumor necrosis factor inhibitors (adalimumab and certolizumab). Details of therapy for Crohn disease were not available for the other patient (case 10). One patient (case 1) had a 3-year history of ulcerative colitis before diagnosis of indolent T-LPD. Another patient (case 6) had a 7-year history of IBD and was treated with adalimumab and certolizumab for a short period. He also had multiple bowel resections with resultant short bowel syndrome and total parenteral nutrition dependence. Because we were unable to obtain all the biopsies on the latter 2 patients (cases 1 and 6), it is unclear if these patients indeed had IBD preceding indolent T-LPD. Six patients were initially diagnosed with PTCL, but the diagnosis was subsequently revised to indolent T-LPD.
Clinical and endoscopic findings and follow-up of 10 patients with indolent T-LPD of the GI tract
| Case . | Age (y)/sex . | Clinical presentation . | Sites of involvement . | Endoscopic findings . | Follow-up (mo) . |
|---|---|---|---|---|---|
| 1 | 15/F | Abdominal pain, diarrhea | Small intestine (jejunum, ileum), colon | Numerous small polyps, erosions | AWD (52) |
| 2 | 31/M | Diarrhea | Small intestine (ileum), colon | Numerous small polyps, erythema | AWD (17) |
| 3* | 35/M | Oropharyngeal ulcers, rectal bleeding | Oral cavity, small intestine (ileum), colon | Colon: oozing erosions | AWD (156) |
| 4 | 38/M | Abdominal pain, diarrhea, food intolerance | Esophagus, stomach, small intestine (duodenum, ileum), colon | Stomach: unremarkable; duodenum: thickened folds | AWD (14) |
| 5 | 46/M | Oral ulcers, abdominal pain, diarrhea; h/o Crohn disease† | Oral cavity, small intestine (ileum), colon | NA | AWD (9) |
| 6 | 50/M | Diarrhea, dyspepsia, drenching night sweats; 7-y h/o IBD | Small intestine (duodenum) | NA | ANED (84) |
| 7 | 52/F | Abdominal pain, vomiting, diarrhea | Stomach | NA | AWD (24) |
| 8 | 52/M | Abdominal pain, bloody diarrhea | Colon | Congestion, erythema and friable mucosa | AWD (175) |
| 9 | 59/F | Abdominal bloating, diarrhea, foul stools; hypocalcemia, hypomagnesemia, hypokalemia and hypozincemia associated with paraesthesias and confusion | Small intestine (duodenum) | “Irregular” appearance of duodenal mucosa | AWD (23) |
| 10 | 77/F | Oropharyngeal ulcers; h/o Crohn disease† | Oral cavity, small intestine (ileum) | NA | AWD (168) |
| Case . | Age (y)/sex . | Clinical presentation . | Sites of involvement . | Endoscopic findings . | Follow-up (mo) . |
|---|---|---|---|---|---|
| 1 | 15/F | Abdominal pain, diarrhea | Small intestine (jejunum, ileum), colon | Numerous small polyps, erosions | AWD (52) |
| 2 | 31/M | Diarrhea | Small intestine (ileum), colon | Numerous small polyps, erythema | AWD (17) |
| 3* | 35/M | Oropharyngeal ulcers, rectal bleeding | Oral cavity, small intestine (ileum), colon | Colon: oozing erosions | AWD (156) |
| 4 | 38/M | Abdominal pain, diarrhea, food intolerance | Esophagus, stomach, small intestine (duodenum, ileum), colon | Stomach: unremarkable; duodenum: thickened folds | AWD (14) |
| 5 | 46/M | Oral ulcers, abdominal pain, diarrhea; h/o Crohn disease† | Oral cavity, small intestine (ileum), colon | NA | AWD (9) |
| 6 | 50/M | Diarrhea, dyspepsia, drenching night sweats; 7-y h/o IBD | Small intestine (duodenum) | NA | ANED (84) |
| 7 | 52/F | Abdominal pain, vomiting, diarrhea | Stomach | NA | AWD (24) |
| 8 | 52/M | Abdominal pain, bloody diarrhea | Colon | Congestion, erythema and friable mucosa | AWD (175) |
| 9 | 59/F | Abdominal bloating, diarrhea, foul stools; hypocalcemia, hypomagnesemia, hypokalemia and hypozincemia associated with paraesthesias and confusion | Small intestine (duodenum) | “Irregular” appearance of duodenal mucosa | AWD (23) |
| 10 | 77/F | Oropharyngeal ulcers; h/o Crohn disease† | Oral cavity, small intestine (ileum) | NA | AWD (168) |
ANED, alive no evidence of disease; AWD, alive with disease; h/o, history of; IBD, inflammatory bowel disease; NA, not available.
Previously published case.9
Biopsies diagnosed as Crohn disease were not reviewed by authors.
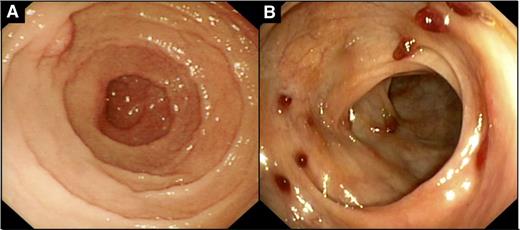
The lesions involved all GI sites, with small intestine (8 patients) and colon (6 patients) being the most commonly involved sites, followed by oral cavity (3 patients), stomach (2 patients), and esophagus (1 patient). Four patients had involvement of only 1 site, whereas multiple sites were involved in 6 patients. The endoscopic findings were available in 6 cases and were not uniform. Upper endoscopy was performed in 2 patients (cases 4 and 9), and in 1 case, although microscopically involved by indolent T-LPD, stomach appeared grossly normal but the duodenum showed thickened folds. In the other case (case 9), the duodenal mucosa showed an “irregular” appearance (Figure 1A). In the colon, numerous small polyps with associated mucosal erythema were found in case 2 (Figure 1B). Furthermore, endoscopic findings in the colon included numerous small polyps with associated erosions in case 1, oozing erosions in case 3, and congestion, erythema, and friable mucosa in case 8. The results of the imaging workup—computed tomography or positron emission tomography scans—were available in 7 patients. In 4 patients, there was no evidence of lymphadenopathy, organomegaly, or masses. In 1 patient (case 1), positron emission tomography scan showed low to intermediate fluorodeoxyglucose uptake in regional lymph nodes. Computed tomography scan showed mildly enlarged mesenteric lymph nodes and mild splenomegaly in 1 patient (case 3), whereas in another patient (case 9), computed tomography showed several mildly enlarged mesenteric and para-aortic lymph nodes. Lymph node biopsies were not performed in either of these cases. Bone marrow examination was performed in 7 patients and was negative in all cases for involvement by T-LPD. Flow cytometry was available in 3 staging bone marrows and in all cases it showed no evidence of abnormal T-cell population. However, in case 2, the peripheral blood showed mild increase in CD8-positive T cells, and TCR-γ chain gene rearrangement revealed a small clone identical to the 1 in the colonic biopsy.
Endoscopic findings in indolent T-LPD of the GI tract. (A) Irregular appearance of duodenal mucosa (case 9). (B) Multiple small polyps with mucosal erythema in the colon (case 2).
Endoscopic findings in indolent T-LPD of the GI tract. (A) Irregular appearance of duodenal mucosa (case 9). (B) Multiple small polyps with mucosal erythema in the colon (case 2).
Clinical management and follow-up
The median follow-up time was 38 months (range, 9-175 months). Four patients were managed conservatively by a “watch and wait” approach and received no chemotherapy. Three of these 4 patients were alive with persistent disease at the last follow-up, and 1 patient (case 6), who had multiple small bowel resections for a diagnosis of IBD, showed no evidence of residual disease at last follow-up. Because of the initial diagnosis of PTCL, 5 patients were treated with chemotherapy including 8 cycles of romidepsin and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and 6 cycles of dexamethasone, aracytine, and oxaliplatin (case 2); 5 cycles of CHOP (case 5); 4 cycles of CHOP (case 8); and multiple cycles of unknown chemotherapy (case 7). One patient (case 1), who was initially diagnosed with ulcerative colitis, underwent colectomy 5 months before the diagnosis of PTCL, which was later revised to indolent T-LPD. More than 3 years after the diagnosis of PTCL, she received 3 cycles of CHOP chemotherapy. One patient (case 10) was diagnosed with PTCL in the oral cavity 13 years before a diagnosis of indolent T-LPD was made in the small intestine; however, details of the clinical management for the PTCL were unavailable. The biopsy of the oral cavity from 13 years prior was reviewed and the diagnosis was revised to indolent T-LPD. Three patients (cases 1, 2, and 8) had no response to chemotherapy and the decision was made to stop the treatment and follow them closely. All 3 were alive with persistent disease at last follow-up. Another patient (case 7) also had no significant response to chemotherapy, but was lost to follow-up after 24 months. One patient (case 5) had significant improvement of the clinical symptoms with complete resolution of oral ulcers and diarrhea after 5 cycles of CHOP. However, oral lesions in this patient showed spontaneous clinical improvement even before the chemotherapy was administered. None of the 10 patients had progression or transformation to an aggressive lymphoma.
Histologic findings
GI biopsies in all 10 patients showed similar morphologic features. In the stomach, small intestine, and colon, the lamina propria was expanded by a dense, nondestructive lymphoid infiltrate that occasionally extended into the muscularis mucosae and submucosa. The mucosal glands were displaced and distorted by the infiltrate (Figure 2A-B). One case (case 9) involving the duodenum showed an increase in villous intraepithelial lymphocytes (Figure 3A). However, there was no significant infiltration of colonic crypt epithelium or surface epithelium by lymphoid cells. The infiltrates were composed of predominantly small, monotonous lymphoid cells with slightly irregular nuclei, mature nuclear chromatin, inconspicuous nucleoli, and scant pale cytoplasm (Figures 2C and 3B). Occasional eosinophils were intermixed with the lymphoid cells. The oral lesions manifested as ulcers with an underlying lymphoid infiltrate involving the mucosa that was similar in appearance to the infiltrate in other GI sites. Occasional cases had small B-cell nodules at the periphery of the infiltrate.
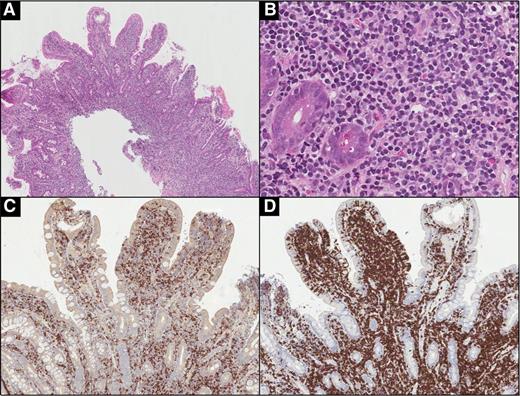
Indolent T-LPD of the ileum (case 10). (A) A resection specimen showing diffuse, band-like infiltration of the lamina propria, and, focally, the submucosa by lymphoid infiltrate. (B) Lymphoid infiltrate distorts and displaces intestinal crypts, but does not invade them. (C) The infiltrate is composed of small, mature-appearing lymphoid cells with scant pale cytoplasm. (D) The cells are positive for TIA-1. The majority of the cells are positive for (F) CD8, with fewer (E) CD4-positive cells admixed. (A: hematoxylin and eosin [H&E], original magnification ×10; B: H&E, original magnification ×100; C: H&E, original magnification ×400; D: immunohistochemical stain, original magnification ×400; E-F: immunohistochemical stain, original magnification ×100.)
Indolent T-LPD of the ileum (case 10). (A) A resection specimen showing diffuse, band-like infiltration of the lamina propria, and, focally, the submucosa by lymphoid infiltrate. (B) Lymphoid infiltrate distorts and displaces intestinal crypts, but does not invade them. (C) The infiltrate is composed of small, mature-appearing lymphoid cells with scant pale cytoplasm. (D) The cells are positive for TIA-1. The majority of the cells are positive for (F) CD8, with fewer (E) CD4-positive cells admixed. (A: hematoxylin and eosin [H&E], original magnification ×10; B: H&E, original magnification ×100; C: H&E, original magnification ×400; D: immunohistochemical stain, original magnification ×400; E-F: immunohistochemical stain, original magnification ×100.)
Indolent T-LPD of the duodenum (case 9). (A) A dense infiltrate involving the lamina propria, muscularis mucosae, and submucosa. (B) The infiltrate is composed of small lymphoid cells with slightly irregular nuclei and occasional admixed eosinophils. Immunohistochemical stains for (C) CD4 and (D) CD8 show the lymphoid cells to be predominantly CD8-positive and focally involve the villous epithelium. (A: H&E, original magnification ×40; B: H&E, original magnification ×400; C-D: immunohistochemical stain, original magnification ×100.)
Indolent T-LPD of the duodenum (case 9). (A) A dense infiltrate involving the lamina propria, muscularis mucosae, and submucosa. (B) The infiltrate is composed of small lymphoid cells with slightly irregular nuclei and occasional admixed eosinophils. Immunohistochemical stains for (C) CD4 and (D) CD8 show the lymphoid cells to be predominantly CD8-positive and focally involve the villous epithelium. (A: H&E, original magnification ×40; B: H&E, original magnification ×400; C-D: immunohistochemical stain, original magnification ×100.)
Immunohistochemistry and molecular studies
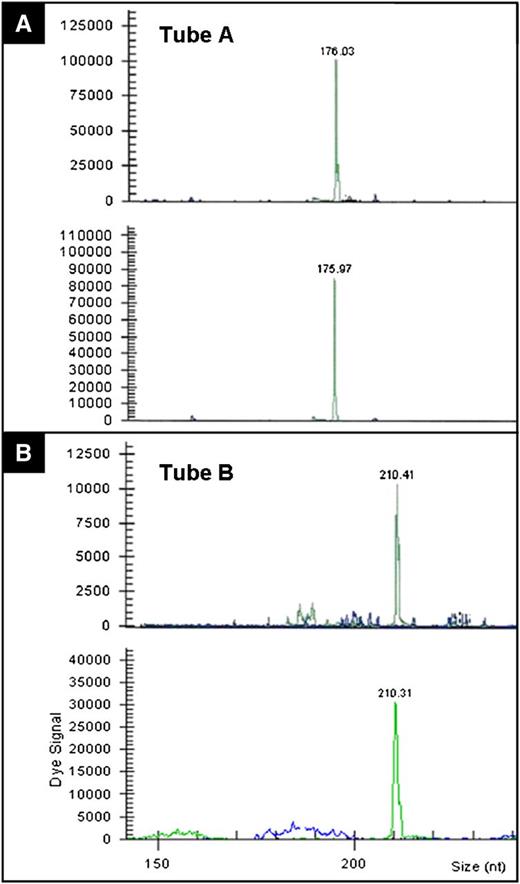
The immunophenotypic and molecular findings are summarized in Table 2. In 8 of the 10 cases, the lymphoid cells were CD8-positive/CD4-negative (Figures 2E-F and 3C-D). Furthermore, in all 8 of these cases, in which immunostains were performed, the cells expressed CD2, CD3, CD5, TIA1, and TCR-BF1 (Figure 2D). One case was also positive for granzyme B. Loss of CD7 was seen in 2 cases, and the cells were negative for CD56 and TCR-G. The proliferative index, demonstrated by Ki67 stain, was exceptionally low (5%) in all cases. One of the 10 cases was CD4-positive/CD8-negative and expressed CD2, CD3, and TCR-BF1, whereas another case was CD4/CD8-double negative and expressed CD2, CD3, CD7, and TCR-BF1. CD30 immunostain was performed in 8 cases and they were all negative. EBER in situ hybridization was performed in 8 cases and was negative in all of them. The molecular studies for a TCR-γ chain gene rearrangement showed a clonal rearrangement in all 10 cases. In case 10, parallel studies were performed on both intestinal and oral lesions (which was diagnosed as PTCL 13 years prior), and showed identical rearrangements (Figure 4A-B).
Immunohistochemical and molecular findings in 10 cases of indolent T-LPD of the GI tract
| Case . | CD3 . | CD4/CD8 . | CD2 . | CD5 . | CD7 . | CD30 . | CD56 . | TIA1 . | GRZB . | TCR-BF1 . | TCR-G . | Ki67, % . | EBER . | TCR-PCR . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | −/+ | + | + | + | − | − | + | − | + | NA | 5-10 | − | Clonal |
| 2 | + | −/+ | + | + | + | − | − | + | − | NA | NA | 5-10 | − | Clonal |
| 3 | + | −/+ | NA | + | NA | NA | − | + | − | + | − | NA | − | Clonal |
| 4 | + | −/+ | + | + | + | − | − | + | NA | + | − | 5 | − | Clonal |
| 5 | + | −/− | + | − | + | − | − | − | NA | + | − | NA | − | Clonal |
| 6 | + | +/− | + | − | − | − | − | − | − | + | − | NA | NA | Clonal |
| 7 | + | −/+ | NA | + | − | NA | − | + | − | NA | NA | 5 | − | Clonal |
| 8 | + | −/+ | NA | + | − | − | − | + | − | + | − | 5 | − | Clonal |
| 9 | + | −/+ | + | + | + | − | − | NA | − | NA | NA | 5 | NA | Clonal |
| 10 | + | −/+ | + | + | + | − | − | + | + | + | NA | 5 | − | Clonal |
| Case . | CD3 . | CD4/CD8 . | CD2 . | CD5 . | CD7 . | CD30 . | CD56 . | TIA1 . | GRZB . | TCR-BF1 . | TCR-G . | Ki67, % . | EBER . | TCR-PCR . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | −/+ | + | + | + | − | − | + | − | + | NA | 5-10 | − | Clonal |
| 2 | + | −/+ | + | + | + | − | − | + | − | NA | NA | 5-10 | − | Clonal |
| 3 | + | −/+ | NA | + | NA | NA | − | + | − | + | − | NA | − | Clonal |
| 4 | + | −/+ | + | + | + | − | − | + | NA | + | − | 5 | − | Clonal |
| 5 | + | −/− | + | − | + | − | − | − | NA | + | − | NA | − | Clonal |
| 6 | + | +/− | + | − | − | − | − | − | − | + | − | NA | NA | Clonal |
| 7 | + | −/+ | NA | + | − | NA | − | + | − | NA | NA | 5 | − | Clonal |
| 8 | + | −/+ | NA | + | − | − | − | + | − | + | − | 5 | − | Clonal |
| 9 | + | −/+ | + | + | + | − | − | NA | − | NA | NA | 5 | NA | Clonal |
| 10 | + | −/+ | + | + | + | − | − | + | + | + | NA | 5 | − | Clonal |
EBER, Epstein-Barr virus-encoded RNA; GRZB, granzyme B; NA, not available; TCR-BF1, T-cell receptor β F1; TCR-G, T-cell receptor γ; TCR-PCR, T-cell receptor-γ chain gene rearrangement by polymerase chain reaction; +, positive; −, negative.
TCR-γ chain gene rearrangement (case 10). Studies were performed using BIOMED2 primers. TCR-γ chain assessment by (A) tube A and (B) tube B in oral (upper panels on A-B) and intestinal (lower panels on A-B) lesions that show identical gene rearrangement patterns.
TCR-γ chain gene rearrangement (case 10). Studies were performed using BIOMED2 primers. TCR-γ chain assessment by (A) tube A and (B) tube B in oral (upper panels on A-B) and intestinal (lower panels on A-B) lesions that show identical gene rearrangement patterns.
STAT3 mutation status
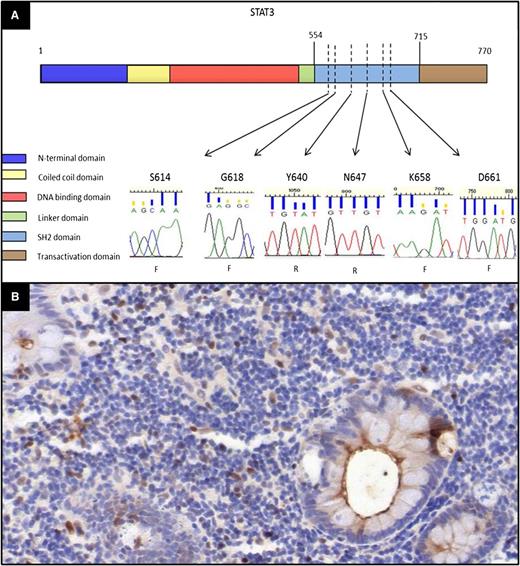
To address whether STAT3 SH2 domain hotspot mutations are present in indolent T-LPD, we evaluated 5 cases (cases 1, 2, 4, 8, and 10) with available material using PCR primers spanning the regions and subsequent sequencing. We did not observe any mutations in the 6 hot spots screened in these cases (Figure 5A).
Evaluation of the mutation status of STAT3 and immunohistochemistry for pY-STAT3 in indolent T-LPD. (A) Sanger sequence chromatograms for STAT3 mutation hot spots showing only wild-type sequences. The amino acid residues corresponding to the sequence evaluated are shown over each chromatogram (F, forward primer sequencing; R, reverse primer sequencing). The domains of the STAT3 protein were shown based on the National Center for Biotechnology Information Reference Sequence NP_644805. The locations of the STAT3 hot spots were indicated based on the STAT3 SH2 domain mutations identified in T-cell and NK-LGLL cases in 2 recent studies.16,17 (B) Immunohistochemical stain for phospho-STAT3 showing only a few scattered positive cells as well as fewer strongly positive cells. (Immunohistochemical stain, original magnification ×200.)
Evaluation of the mutation status of STAT3 and immunohistochemistry for pY-STAT3 in indolent T-LPD. (A) Sanger sequence chromatograms for STAT3 mutation hot spots showing only wild-type sequences. The amino acid residues corresponding to the sequence evaluated are shown over each chromatogram (F, forward primer sequencing; R, reverse primer sequencing). The domains of the STAT3 protein were shown based on the National Center for Biotechnology Information Reference Sequence NP_644805. The locations of the STAT3 hot spots were indicated based on the STAT3 SH2 domain mutations identified in T-cell and NK-LGLL cases in 2 recent studies.16,17 (B) Immunohistochemical stain for phospho-STAT3 showing only a few scattered positive cells as well as fewer strongly positive cells. (Immunohistochemical stain, original magnification ×200.)
Immunohistochemistry for phospho-STAT3
To further investigate STAT3 activation, we performed immunostaining for nuclear pY708-STAT3. The pattern of staining was quite similar in all 5 cases, with a few scattered weakly positive and even fewer strongly positive lymphoid cells accounting for less than 10% of the lymphoid population. There were also occasional foci with more abundant positive cells, which were mostly found in the subepithelial areas or in small mucosal foci that might correspond to follicles. The staining pattern indicated that the vast majority of the infiltrating T cells did not express activated STAT3 (Figure 5B).
Discussion
In this study, we report a unique group of 10 patients with an indolent T-cell proliferation in the GI tract. The majority of patients were young to middle-aged, predominantly males, who commonly presented with abdominal pain and diarrhea in some cases mimicking IBD. The lesions involved all sites of the GI tract, with small intestine and colon being the most commonly involved sites. In 8 cases, the atypical lymphoid cells were CD8-positive/CD4-negative and expressed the cytotoxic marker TIA1. However, the other 2 cases had different phenotypes with 1 case being CD4-positive/CD8-negative and the other double negative for CD4 and CD8. These findings indicate that, even though these indolent proliferations are most commonly CD8-positive, other phenotypes can be observed in a histologically and clinically similar process. Complete clinical staging was performed in 7 patients and 4 had no evidence of systemic involvement. In the other 3 patients, enlarged regional lymph nodes were found but were not biopsied, and the cause of the lymph node enlargement is unclear. Two of these patients never received any therapy and were alive with disease at 23 and 156 months after diagnosis, respectively. The third patient received chemotherapy with no response and is alive with persistent disease 52 months after diagnosis. Interestingly, in 1 patient with negative bone marrow biopsy, a small T-cell clone identical to the clone in a colon biopsy was found in the peripheral blood. This patient had extensive involvement of the colon and ileum by indolent T-LPD; some cells from the intestinal lesions probably entered the circulation and were detected by PCR. However, this was not associated with evidence of disseminated clinical disease.
In 6 cases, the diagnosis of PTCL was initially made and 5 patients underwent chemotherapy, with little or no response in 4. Furthermore, several patients were diagnosed with IBD and treated, also with no response. All patients had a protracted clinical course with persistent disease, but without progression to aggressive lymphoma. The remarkable clinical and morphologic similarities between our cases suggest that these lesions may represent a distinct disease entity of an as-yet unknown etiology. We believe that this disorder is largely unrecognized, with many cases diagnosed as either PTCL or IBD. The clonal TCR gene rearrangement present in all cases certainly contributed to the diagnosis of PTCL, leading to unnecessary aggressive therapy with no significant clinical benefit. In 1 patient, who presented with an oral lesion diagnosed as PTCL and then with small intestinal involvement 13 years later, we detected identical TCR gene rearrangements in both lesions. Larger studies are needed to establish the optimal clinical management of these patients. However, our findings suggest that careful clinical follow-up with minimal therapy might be sufficient. These patients could potentially benefit from therapies such as low-dose methotrexate or cyclosporine A, which have proven to be effective in LGLL.18
Review of the literature revealed 2 small case series and several case reports of indolent T-LPD of the GI tract,5-8,10-13 for a total of 12 reported cases. These cases show clinical and pathological similarities to our series and are summarized in supplemental Table 2. Among the 12 reported patients, there were 10 males and 2 females, with a median age of 50 years (range, 23-67 years). Interestingly, 11 of these cases were CD4-positive, in contrast to our series in which CD8-positive cases predominated. In 1 case, CD4 and CD8 stains were not performed. This bias toward CD4-positive cases reported in the literature suggests that indolent CD8-positive proliferations are more commonly diagnosed as EATL or PTCL. Molecular studies for TCR-γ chain gene rearrangement were performed in 11 cases and showed a clonal rearrangement in 10 cases, whereas 1 case was oligoclonal. Seven patients were given chemotherapy with partial or no response in 6 cases, whereas the lesions resolved in 1 patient. Therapy was less intensive in other 5 patients, largely with no response. After a median follow-up of 56.5 months (range, 12-176 months), 8 patients were alive with persistent disease and 1 had no evidence of disease after chemotherapy. In 1 patient, disease progressed with involvement of mesenteric and retroperitoneal lymph nodes and liver. This patient died 176 months after diagnosis.8 In 1 case, transformation to large T-cell lymphoma occurred 132 months after diagnosis, and the patient died after bowel perforation.13 Indolent T-cell proliferations have also been reported in the skin, specifically affecting the face.19-22 These proliferations most commonly present as nodules on the external ear, are more common in men, and persist if left untreated but do not progress to aggressive lymphoma. By immunohistochemistry, these lesions are also positive for CD8 and TCR-BF1 and express the cytotoxic marker TIA1.19,21,22 Most are clonal by TCR-γ chain gene rearrangement.19-22 A possible relationship of this indolent CD8-positive lymphoid proliferation of the face with indolent T-LPD of the GI tract is uncertain because the etiology of both conditions is unknown.
The differential diagnosis of indolent T-LPD includes EATL, IBD, and celiac sprue, and careful integration of the endoscopic, histologic, and immunophenotypic features is necessary to make the correct diagnosis. EATL most commonly presents with extensive mucosal ulcerations and deep infiltration of the bowel wall. Frequently, there is multifocal involvement as well as infiltration of adjacent abdominal structures and associated ascites.1-3 In contrast, indolent T-LPD usually presents as 1 or more shallow mucosal ulcers with associated erythema or as multiple small polyps. Colonic lesions can be confluent, giving the endoscopic appearance of IBD. Histologically, these lesions are nondestructive, involving mostly the lamina propria and muscularis mucosae, and are composed predominantly of small, mature-appearing lymphoid cells. In contrast, type I EATL is usually a large and destructive lesion composed of medium- to large-sized pleomorphic cells with prominent nucleoli. In type II EATL, the cells are monotonous and small to medium in size, and lesions are also infiltrative and highly destructive. Importantly, type II EATL is characterized by florid infiltration of the intestinal crypt epithelium and adjacent intestinal mucosa by lymphoma cells, whereas indolent T-LPD shows little or no involvement of the crypt or surface epithelium. Furthermore, in type I EATL, the intestinal mucosa adjacent to the main tumor mass frequently shows evidence of enteropathy, whereas none of our T-LPD patients had a history of celiac disease or showed histologic evidence of enteropathy.1-4 Type II EATL has a characteristic immunophenotype, with the tumor cells being positive for CD3, CD8, CD56, and often TCR-G, but negative for CD4.3 In contrast, all of our CD8-positive cases were negative for CD56. The absence of CD56 expression also aids in the distinction from NK cell enteropathy, a condition with a very similar clinical presentation and endoscopic findings.14,15 The immunophenotype of type I EATL can overlap with that of indolent T-LPD. However, the macroscopic appearance of the lesions, the morphology, and destructive nature of the infiltrates as well as the associated enteropathy should all point toward a diagnosis of EATL.
Another differential diagnosis to consider is IBD (primarily ulcerative colitis), and this was the initial diagnosis in several of the cases in this study. Even though some morphologic features, such as crypt distortion, may superficially mimic the changes seen in ulcerative colitis, careful examination reveals no other diagnostic features such as cryptitis, crypt abscesses, reduced intraepithelial mucin, basal plasmacytosis, submucosal fibrosis, hypertrophic muscularis mucosae, and Paneth cell hyperplasia.23 One patient in our series had duodenal involvement with an increase in villous intraepithelial lymphocytes, which raised the differential diagnosis of celiac sprue. However, the distribution of the lymphocytes was not characteristic of celiac sprue (ie, clustering at the tip of the villi), and none of the other features usually seen in celiac sprue (eg, villous atrophy, hyperplastic and elongated crypts, increase in plasma cells in lamina propria) were present.24 Furthermore, the clinical and laboratory findings in this patient were not supportive of celiac sprue since the symptoms did not improve with a gluten-free diet and immunoglobulin A antiendomysial antibodies were absent.
Activating STAT3 mutations affecting the dimerization surface of the SH2 domain have recently been reported in T-cell LGLL.16,17 This disease is another clonal, predominantly CD8-positive, T-cell proliferation with an indolent clinical course, but with a different clinical presentation. Therefore, we investigated the presence STAT3 mutations in our cases but none was found. We also looked for the expression of nuclear pY705-STAT3, which was also absent in our cases. These findings suggest that STAT3 activation is not the underlying mechanism for this indolent T-LPD. However, we were not able to completely exclude this mechanism in our series because we are unable to study all of the cases. Application of next-generation sequencing-based techniques may be useful for the identification of the genes and pathways responsible for this disorder. Furthermore, this T-LPD could potentially be immunologically mediated. Jiang et al25 have recently shown that localized vaccinia virus infection in mice generates long-lived, nonrecirculating CD8-positive resident memory T cells that reside within the skin. Similarly, a persistent antigenic stimulus could potentially initiate indolent T-LPD, in a genetically susceptible individual, with recruitment and eventual clonal evolution of T cells in the GI tract.
In conclusion, we report 10 cases of an indolent T-LPD involving the GI tract. This largely unrecognized condition mimics intestinal T-cell lymphoma and other inflammatory diseases of the GI tract, which can lead to misdiagnosis and unnecessary treatment. It is extremely important for pathologists and clinicians to be aware of this disorder in order to best manage these patients. Any part of the GI tract can be involved; histologic criteria for making the diagnosis include involvement of mucosa/lamina propria by predominantly small, monotonous lymphoid cells with scant cytoplasm. The infiltrate sometimes extends into the submucosa, but does not usually involve the full thickness of the bowel and does not form tumor masses. Lesions can be continuous or multifocal. The immunophenotype is variable, but molecular studies for a TCR-γ chain gene rearrangement invariably show clonal rearrangement. Furthermore, it is important to correlate the histological features with clinical findings and perform complete lymphoma staging to exclude involvement of other sites outside the GI tract, because these lesions appear to be localized to the GI tract in the majority of cases. We propose the term “indolent T-cell lymphoproliferative disease of the GI tract” to emphasize the indolent clinical course of this disorder and clearly separate it from inflammatory bowel disease and the aggressive T-cell lymphomas.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Michael Levy and Jehan Dupuis for providing endoscopic pictures and clinical information, Dr Unn Merete Fagerli for providing clinical information, and Cindy Lachel for technical assistance.
Authorship
Contribution: A.M.P. collected the data and wrote the manuscript; R.A.W., Q.H., P.G., C.C.-B., S.A., H.-Y.W., J.X.C., C.M.B., J.D., E.R., and D.D.W. identified and contributed cases and clinical information; C.K., X.Z.H., and W.C.C. performed laboratory experiments; E.S.J. and W.C.C. identified cases, collected data, and wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest-disclosure: The authors declare no competing financial interest.
The current affiliation for J.X.C. is Department of Pathology, The University of Chicago, Chicago, IL.
Correspondence: Wing C. Chan, Department of Pathology and Microbiology, 983135 Nebraska Medical Center, Omaha, NE 68198-3135; e-mail: jchan@unmc.edu; and Elaine S. Jaffe, Laboratory of Pathology, National Cancer Institute/National Institutes of Health, Bldg 10, Room 2B42, Bethesda, MD 20892; e-mail: ejaffe@mail.nih.gov.
References
Author notes
E.S.J. and W.C.C. contributed equally to this study.

![Figure 2. Indolent T-LPD of the ileum (case 10). (A) A resection specimen showing diffuse, band-like infiltration of the lamina propria, and, focally, the submucosa by lymphoid infiltrate. (B) Lymphoid infiltrate distorts and displaces intestinal crypts, but does not invade them. (C) The infiltrate is composed of small, mature-appearing lymphoid cells with scant pale cytoplasm. (D) The cells are positive for TIA-1. The majority of the cells are positive for (F) CD8, with fewer (E) CD4-positive cells admixed. (A: hematoxylin and eosin [H&E], original magnification ×10; B: H&E, original magnification ×100; C: H&E, original magnification ×400; D: immunohistochemical stain, original magnification ×400; E-F: immunohistochemical stain, original magnification ×100.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/22/10.1182_blood-2013-07-512830/4/m_3599f2.jpeg?Expires=1769079460&Signature=jiIovkIEdh2MIuGqmC3C3iCo5f8-fpS~oplA4FoXsf-0sjxIYtonbqqsalpV7e2-mNJUXT3rI9SiCOo9ImflqviGoL6r9xSboNh9lU3FZFP8NYO1ZX43biGJFa4529iI70btnUR35IIFlTkehZemCUJgDzgBhgFvPelRM~vj-cVdiw8Zpa6B~xWVBdZ8TowQ~Qeb9306ZYndqOdAQa2Qg-pp41De-hdWUb-zWhOOA-7U7kFSqXTPLGJB8bTLXjFjewbsKwkM7VqlHUzAjdRqw~zxOLvy0veBLI3Mhwi2mwTWYNNaKr0Kjw3prwqP-NEFkLSFVMMuOpAsMNcaAzsPAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal