In this issue of Blood, Leveson-Gower et al describe an immunomodulatory role of mast cells in graft-versus-host-disease (GVHD).1
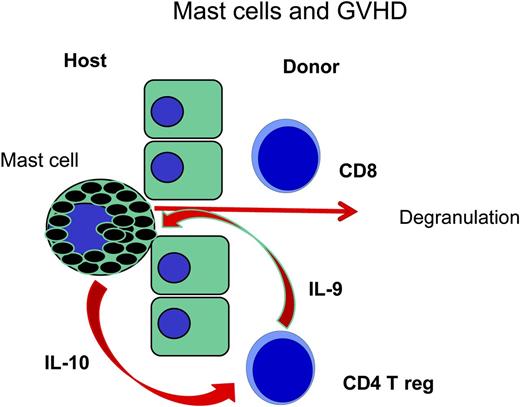
In an early step of GVHD an accumulation of CD8 T cells and mast cells is observed in the host's epithelium; following degranulation, activated mast cells produce IL-10 to stimulate regulatory CD4 T cells which in turn may produce IL-9 for the stimulation of mast cells. In mast cell deficient mice (C57BL/6-KitW-sh/Wsh) regulatory CD4 T cells may not be sustained and other T cells may proliferate in an overwhelming fashion (see Lu et al7 ).
In an early step of GVHD an accumulation of CD8 T cells and mast cells is observed in the host's epithelium; following degranulation, activated mast cells produce IL-10 to stimulate regulatory CD4 T cells which in turn may produce IL-9 for the stimulation of mast cells. In mast cell deficient mice (C57BL/6-KitW-sh/Wsh) regulatory CD4 T cells may not be sustained and other T cells may proliferate in an overwhelming fashion (see Lu et al7 ).
GVHD is the major obstacle to allogeneic stem cell transplantation, and its pathophysiology is being increasingly analyzed.2 There is little doubt that T cells are the effector cells, and histocompatibility antigens are the targets against which they are directed. However, how the damage is caused in the target organs, skin, gut, and liver is still under debate. It is not known why some patients still develop severe GVHD under immunosuppressive treatment and others do not. Neither histocompatibility differences nor composition of the graft allows a precise prognosis of GVHD. Mechanisms of modulation of the immune response have come into focus; innate immunity with release of cytokines, antigen presentation, stimulation and expansion of T cells, involvement of naïve and memory T cells, effector T cells, and their effector mechanisms including recruitment of other cells such as B cells, macrophages, and others play important roles. Nevertheless, immune tolerance may be accomplished by the same mechanisms that prevent immune damage in situations other than transplants. This tolerance may be a “cease fire” by mechanisms mediated by programmed death 1/programmed death 1-ligand ligation, as well as B7/cytotoxic T lymphocyte antigen 4 binding. Several forms of regulatory immune cells have been described.4 Regulatory T cells that have been characterized by Edinger et al3 and others as CD4+ T cells. However, CD25 high positive and FOX P3 positive, or other cells like myeloid derived suppressor cells, CD8 positive suppressor cells and veto cells also have immunomodulatory functions.4 Their suppressive activity may be direct by cell-cell contact or via dendritic cells modulating T cells. In this article, a new form of immunomodulation has been proposed: suppression of GVHD via the secretion of interleukin (IL)-10 by mast cells.1
Mast cells were first described by Ehrlich in his doctoral thesis5 ; he suspected a nutritive role of the granula of the mast cells for other cells in bone marrow. In the mean time, we learned that mast cells contain many biologically active substances such as histamine, heparin, proteases, and cytokines. These have a beneficial role in the immune control of worm infestations; their role in allergies after binding IgE and immune complexes is less beneficial. Mast cells contain many different biologically active substances; various functions have been attributed to mast cells in different organs as the mucosa, the skin and the connective tissue. As part of innate immunity, they have a stimulatory role; as part of adaptive immunity, they have both modulatory and effector functions. It has become evident that they also play a role in the modulation of T-cell responses. Mast cells may stimulate T cells by secreting tumor necrosis factor α, and may also down-regulate T cells by secreting IL-10.1 Mice with a partial knockout of KitW-sh/Wsh are not anemic but are deficient in functional mast cells; these mice also have more GVHD as shown by the accumulation of luciferase-positive donor T cells of the FVB strain in the gut, the cervical lymph nodes, and the liver. More severe GVHD seen in C57BL/6-KitW-sh/Wsh mice was due to increased proliferation of donor T cells. However the addition of regulatory T cells improved the survival of the animals compared with that of animals given only conventional T cells. The injection of cultured bone marrow mast cells (BMCMCs) into the abdomen could improve survival. This improvement was not as evident in mice given IL-10–deficient BMCMCs. The paper raises important questions regarding the role of mast cells in GVHD. Previous papers on murine GVHD of the skin show a prominent role of CD8 T cells and mast cells, the mast cells being degranulated as early as 7 days after transplantation6 ; CD4 T cells and natural killer cells follow these early events. Lu et al showed that mast cells are necessary7 for the maintenance of tolerance toward skin transplants, and regulatory T cells produce IL-9 as an activation factor for mast cells. In this study, IL-10 produced by mast cells supports tolerance maintenance by regulatory T cells, thereby closing a cycle of tolerance.
This most interesting mechanism of maintenance of tolerance would have been more convincingly demonstrated, if a control group of C57BL/6-KitW-sh/Wsh would have been included that received T cell–depleted marrow and survived, since survival was an endpoint of the experiments. Of course the suspected “cycle of tolerance” could possibly have been demonstrated by the combination of BMCMCs and regulatory T cells. It remains to be shown whether this cycle of tolerance can be demonstrated in a GVHD model and how it may be handled.
Conflict-of-interest disclosure: The author declares no competing financial interests.