Abstract
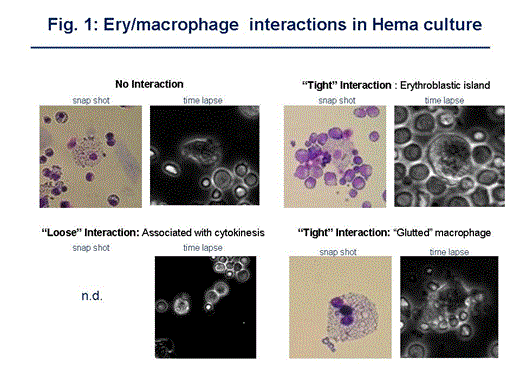
In the marrow, macrophages form a morphological unit, the erythroblast island and in culture release factors still to be characterized that affect the growth of the erythroblasts (Ery). When blood CD34pos cells are stimulated with dexamethasone (Dex) and growth factors in human erythroid massive amplification (HEMA) cultures, large numbers of Ery are generated which remain immature and proliferate for 10-17 days. Dex withdrawl and erythropoietin (EPO) exposure induce Ery to activate the maturation program within 24-48 h. At day 13-14 of HEMA, but not in cultures lacking Dex, rare (0.5-3%) clusters resembling erythroid islands are detectable by morphological and FACS analyses (high Forward Side Scatter CD14/CD36 positive events expressing CD235a at high levels). The role of macrophages in HEMA has not been investigated. Live contrast-phase microscopy (10x magnification; time-lapses: 1 frame/30” recorded for 8-10h) was used to visualize interactions between Ery and macrophages in day 13-14 HEMA with or without Dex and in cultures in which day 13-14 progeny were exposed to EPO alone. Macrophages and Ery at different stages of maturation (proEry, Baso/PolyEry and OrthoEry were recognized on the basis of size, chromatin condensation state, motility and frequency using data previously obtained by FACS as comparison.
Day 14 without Dex
Ery at all maturation stages were observed moving in a coordinate fashion but with frequent changes in directions. Ery formed few, loose and unstable aggregates. Macrophages were rare and moved independently from Ery forming occasional tight interactions lasting for up to 30' with Baso/PolyEry. Cytokinetic events were also rare (0.64 events/h), lasted ∼15' and involved exclusively single proEry not interacting with macrophages. Exposure to EPO alone did not change the interactions between Ery and macrophages.
Day 14 with Dex
Ery (mostly proEry) moved synchronously toward each other forming large and stable aggregates. Macrophages were “fat”, highly motile and extended short protrusions which established transient contacts with hundreds/thousands of Ery over 1 h. Contacts involved both isolated Ery (rapid touch-and-go interactions, “loose”) or clusters of Ery at all maturation stages (interactions that persisted for 10', “tight”). In the first 45' of observation, ∼45% of the interactions were loose, ∼23% were a combination of loose and tight and ∼35% were tight. By 1-2h, 25% were loose, 50% were loose and tight and 25% were tight. Cytokinesis involved single and double proEry. Cytokinesis of single proEry had a frequency of 1.1 event/h, lasted ∼15' and were macrophage independent. Cytokinesis of proEry doublets had a frequency of (0.12 event/h), lasted 15'+15' during which each Ery divided in succession generating four proEry that remained associated for some time and occurred in proximity of a macrophage that gently interacted with the two proEry “touching and releasing” them with “cuddling” movements. Enucleation events were rare and involved an ortho-Ery surrounded by pro-Ery and basoEry. The process lasted 5' and was not associated with macrophages.
Dex withdrawal and EPO exposure induced noticeable changes. By 15', macrophages became greatly motile engaging in fewer “loose” interactions (5-9%) and in more “tight” interactions. By 60', 86% of the interactions were “tight” and involved preferentially clusters of mature Ery (>80%). Mega-aggregates including 2-6 macrophage-Ery clusters each were also observed. Cytokinetic events were not recorded in 2 h of observation of cultures deprived of Dex and exposed to EPO alone.
Time lapse recordings document the occurrence of at least two types of interactions between macrophages and Ery in HEMA: 1) tight interactions involving clusters of mature Ery leading to the formation of structures resembling “erythroid islands”, the frequency of which was greatly increased by Dex withdrawal and exposure to EPO alone; 2) previously undescribed loose interactions involving Ery doublets, associated with cytokinetic events and occurring only in cultures containing Dex that may promote proliferation in HEMA culture.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal