Abstract
Eltrombopag (EP) is a small-molecule, nonpeptide thrombopoietin receptor (TPO-R) agonist which has been shown in-vitro to inhibit leukemia cell growth. The underlying mechanism is still under investigation.
We report a patient with NPM1 mutated/FLT3 negative refractory AML who achieved a complete remission during treatment with single agent EP within the PMA112509 trial. In this patient we conducted sequential molecular analyses out of the bone marrow to study the underlying molecular mechanisms. Therefore, samples prior to EP, at remission and relapse were subjected to genome-wide copy number analysis using Affymetrix SNP 6.0 array in search for acquired copy number alterations (CNA). To screen for alterations in commonly mutated genes in AML, samples were further subjected to a next generation deep sequencing assay (NGS) of mutational hotspots in the genes ASXL1, CBL, DNMT3A, ETV6, EZH2, IDH1/2, KRAS, NPM1, NRAS, RUNX1, SF3B1, SRSF2, TET2, TP53, U2AF1 and ZRSR2. Sequencing was performed on the 454 GS Junior platform (Roche applied science).
Moreover we investigated the expression of TPO-R (CD110) by different assays in cell lines and primary AML samples. To study the TPO-R dependency of potential antineoplastic EP effects we studied the effects of lentiviral TPO-R knockdown and single agent EP on the vitality and cell cycling of TPO-expressing and non-expression leukemia cell lines.
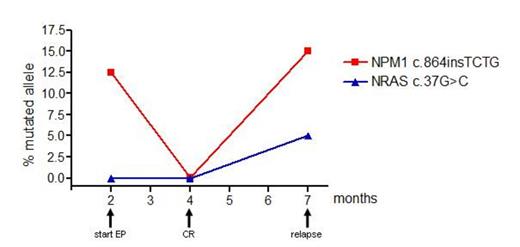
By using NGS we followed the NPM1+ mutation (NPM1 c.864incTCTG) load in this patient and found a concomitant decline (prior EP: 12.6%, at CR: 1.1%) but not disappearance of NPM1+ cells and a reemergence (15.2%) together with a clonal evolution and development of a NRAS c.37G>C mutation during disease progression (Figure 1) while a SNP-array demonstrated no additional CNA at disease progression. Real time PCR analysis demonstrated TPO-R expression at all time points analyzed which declined during complete remission(TPO-R/GAPDH: prior EP: 56.7%, at CR: 32.3%).
These results prompted us to study TPO-R expression of blasts by flow cytometry in de novo AML samples (n=72) at diagnosis. In fact, TPO-R was expressed only in 33 of 72 AML patients but across all FAB and cytogenetic subgroups. The median MFI (mean fluorescence intensity) of CD110 was 2-fold higher on blasts than on CD110 positive lymphocytes. Interestingly, there were some differences with regards to the mutational status, since the NPM mutation was documented more frequently in CD110 negative than in CD110 positive AML cases (26% vs. 10%). These data were confirmed by Taqman-PCR in an independent cohort (n=57) with a nearly three fold lower expression of TPO-R on NPM1+/FLT3- compared to NPM1-/FLT3- (p=0.0163) cases.
Next, we sought to clarify if TPO-R expressing AML cell lines are dependent on TPO-R expression. Knockdown of TPO-R by lentivirally transferred shRNA resulted in down-regulation and rapid cell death in the TPO-R expressing megakaryoblastic cell line (CMK). However, treatment with EP in-vitro at doses ranging from 1 to 10 µg/ml lead to a dose-dependent decrease in the cell division rate and vitality not only in CMK but also in cell-lines with weak or absent surface TPO-R expression (e.g. KG1a, a human acute myeloid leukemia cell line or OCI-AML3, a NPM1+ myeloid cell line). In parallel, a significant counterregulatory upregulation of TPO-R mRNA was observed which was dose-dependent (KG1a, p=0.0014).
These data demonstrate that TPO-R is heterogeneously expressed across all AML subtypes but absent in the majority of NPM1+/FLT3- cases. The clinical response seen in our patient with a refractory NPM1+ AML further provides evidence to the fact that single agent EP can exert potent anti-leukemic effects in-vivo. These effects seem to be mediated rather independently of TPO-R expression.
Platzbecker:GlaxoSmithKline: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal