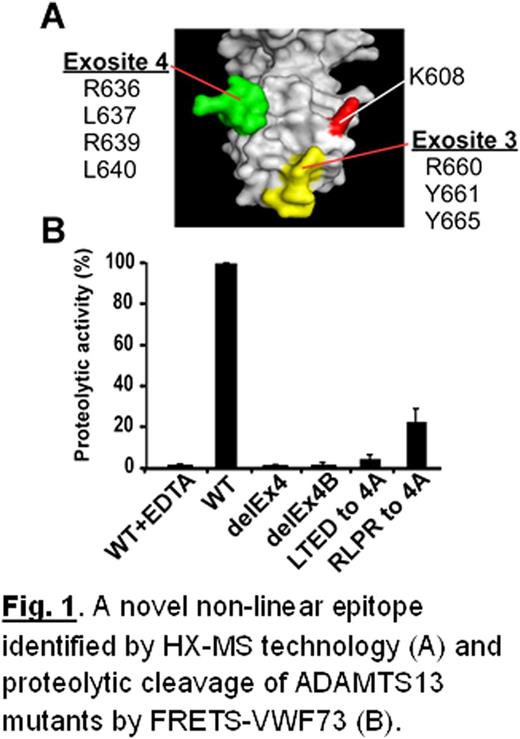
Acquired thrombotic thrombocytopenic purpura (aTTP), a potentially fatal syndrome, is primarily caused by autoantibodies against the metalloprotease ADAMTS13. Most patients with aTTP harbor an immunoglobulin (Ig) G isotype in blood that targets the spacer domain of ADAMTS13. The precise epitopes of the anti-ADAMTS13 IgGs and the mechanism underlying their inhibition activity are not fully understood. We hypothesized that inhibitory IgG autoantibodies from aTTP patients achieve their inhibitory function by binding to a discontinuous epitope in the spacer domain of ADAMTS13. To test this hypothesis, we determined the binding epitope of one out of >100 unique human monoclonal antibody (mAb) fragments (single-chain Fv, scFv) isolated by phage display from aTTP patients. We developed a novel hydrogen-deuterium exchange-mass spectrometry technology (HX-MS) to identify the antibody binding sites at single amino acid residue resolution. Human ADAMTS13 inhibitory scFv 4-20 was expressed in E. coli Top10 cells and purified to homogeneity by Ni-chelating affinity chromatography. In the HX-MS experiment, the mAb was coupled to affi-gel 10 resin and used to bind recombinant ADAMTS13-MDTCS fragment expressed in a stably transfected Drosophila schneider 2 (S2) cell line. After exchange with deuterium (D2O) oxide for various periods of time, the reaction was stopped, the protein was eluted, and digested to peptide fragments with pepsin, and the peptides with or without deuterium bound were resolved and identified by fast HPLC and mass spectrometry. We find that mAb scFv4-20 binds to amino acid residues Arg636, Leu637, Arg639, and Leu640 spanning from Leu632 to Leu640 (in exosite 4) in the spacer domain of ADAMTS13. This sequence is highly conserved in the ADAMTS13 spacer domains from zebrafish to mammals. In addition, mAb scFv4-20 binds Arg660, Tyr661, and Tyr665 in exosite 3, previously shown to play an important role in substrate recognition and anti-ADAMTS13 autoantibody-mediated inhibition, as well as Lys608, upstream exosites 3 and 4. Apparently, mAb scFv4-20 inhibits plasma ADAMTS13 activity (IC50 ∼0.40 nM) by binding these non-linear surface residues in the spacer domain (Fig. 1A). In agreement, site-directed mutagenesis shows that complete deletion (Δ632LTEDRLPR639) or partial deletion (Δ632LTED635 or Δ636RLPR639), or replacement of these residues with alanines (632LTED635/4A or 636RLPR639/4A) abolished or dramatically reduced mAb scFv4-20 binding. A deletion or alanine substitution of the surface residues on exosite 4 also abolished or reduced ADAMTS13 proteolytic activity toward a fluorescein-labeled VWF73 peptide and multimeric VWF (Fig. 1B), indicating that the ADAMTS13 epitope for mAb scFv4-20 is also part of ADAMTS13’s substrate recognition site. We conclude that anti-ADAMTS13 autoantibodies work by physically blocking the well-conserved VWF binding site on ADAMTS13. These results demonstrate the powerful use of HX-MS technology to determine both linear and non-linear antibody binding epitopes. The results provide valuable information concerning the mechanism of autoantibody-mediated aTTP that may be exploited to develop targeted therapy by reengineering ADAMTS13 to avoid autoantibody inhibition.
Disclosures:
No relevant conflicts of interest to declare.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal