T-cell replete haploidentical stem cell transplantation (HaploSCT) using post-transplant cyclophosphamide (PTCY) has been performed primarily with using non-myeloablative conditioning. This approach has been associated with very low non-relapse mortality (NRM); however, a high relapse rate was noted for pts in remission, suggesting that a more intense conditioning could be used to decrease rate of relapse post-transplant. We explored a myeloablative yet reduced-intensity melphalan-based conditioning regimen in an ongoing phase II clinical trial.
Results from the first 57 patients (pts) with advanced hematologic malignancies between 03/2009 and 4/2013 are reported. All pts received a melphalan-based conditioning regimen using fludarabine 160mg/m2, melphalan 140mg/m2 (N=35) or 100mg/m2 (N=22) +/- thiotepa 5-10mg/kg. GVHD prophylaxis was with post transplant cyclophosphamide 50 mg/kg on days +3 and +4 followed by tacrolimus and mycophenolate, for at least 6 and 3 mo post-transplant, respectively. Overall survival (OS) and progression-free survival (PFS) were assessed using the Kaplan-Meier method and group differences were determined using the log-rank test. Cumulative incidence (CI) of relapse, GVHD, and non-relapse mortality (NRM) were assessed using the competing risks method, where the competing risk for CIR was death, for NRM was relapse, and for GVHD was death and relapse. Group differences were determined using Gray's test.
34 (60%) pts were males and the median age was 47 years (range 21-66). Diagnoses were AML/MDS 28 (49.1%), CML 7 (12.3%), ALL 7 (12.3%), and lymphoma/CLL 10 (3 Hodgkin's, 3 NHL, 4 CLL) (17.5%), myelofibrosis/AML 4 (7%) and myeloma 1 pts. Racial distribution was: 18 (31.6%) African-Americans, 16 (28%) Caucasians, 12 (21%) Hispanics, 4 (7%) Asians, 7 (12.3%) Middle-Eastern. All patients except 2 (96.5%) had a bone marrow graft. 20/28 (71.4%) pts with AML/MDS were in complete remission (CR) at transplant (CR1+CR2) and 15/28 pts with AML had poor-risk by cytogenetics (N=12) or FLT3 mutation (N=3). All ALL pts had disease beyond CR1 except 1; 7/10 (70%) lymphoma/CLL pts had advanced disease. All patients with myelofibrosis have progressed to AML. Donors were children (N=26, 46.5%), siblings (N=23, 40.3%), parents (N=7, 12.3%), cousin (N=1), with 3-5 HLA antigen mismatches.
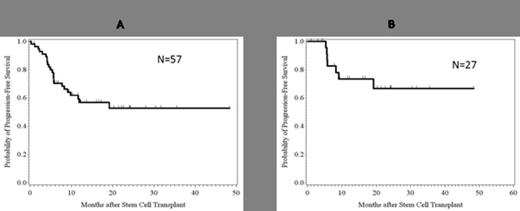
The median follow-up of survivors was 14 mo (range: 1.0-48.4 months). One patient had early death due to RSV infection. Primary engraftment was achieved in 54/56 (96.5%) of evaluable patients, with majority (N=51, 90%) achieving full donor chimerism. Of the 6 who had mixed chimerism, 2 relapsed and 4 pts converted to full chimerism. Median time to neutrophil engraftment was 18 days (range 11-43). The CI of gr 2-4 aGVHD and 3-4 aGVHD were 29.7% and 3.7%, respectively, while CI of cGVHD limited+extensive and extensive only were 23.7% and 11.5%. Overall, the NRM was 21.4%, CI of relapse was 25.8% and PFS was 52.8% at the last follow-up (Figure 1A). NRM for myeloid pts transplanted in remission was 9% and PFS 66.8% at 50 mo median follow-up (Table 1, Figure 1B).
A. PFS all patients; B. PFS for myeloid patients in remission (AML/MDS, CML – chronic phase).
A. PFS all patients; B. PFS for myeloid patients in remission (AML/MDS, CML – chronic phase).
Melphalan-based conditioning for HaploSCT offers good disease control with acceptable NRM for patients in remission at transplant. A low relapse rate was observed in lymphoma/CLL patients despite the fact that most patients were not in remission at transplant. Overall, patients with advanced disease remain a challenge due to higher relapse rate and novel strategies are needed to significantly improve outcomes of these patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal