Abstract
Myelofibrosis (MF) is characterized by progressive bone marrow (BM) fibrosis and ineffective, extramedullary hematopoiesis resulting in splenomegaly, debilitating symptoms, and shortened survival. So far, conventional pharmacotherapy had limited success in improving BM fibrosis. The JAK1/JAK2 inhibitor ruxolitinib (RUX) reduced splenomegaly and symptom burden and improved survival in patients (pts) with intermediate-2 or high-risk MF compared with placebo or best available therapy (BAT) in two phase 3 studies. We examined the effects of long-term RUX treatment on BM morphology in pts with MF from the MD Anderson Cancer Center enrolled in the phase 1/2 study (NCT00509899) and compared the results with a cohort of pts treated with BAT.
Trephine biopsies of pts with primary MF (PMF), post-polycythemia vera MF, or post-essential thrombocythemia were obtained at baseline and at 24 (68 pts), 48 (38 pts), 54 (24 pts), 60 (10 pts) and 66 (4 pts) months (mo) of therapy. World Health Organization (WHO)-defined BM fibrosis grade (scale 0-3) was assessed independently by two authors (JT and HMK) and reviewed by a third author (CB-R). Final grade was determined by consensus, with all reviewers blinded to pt characteristics and outcomes. Accuracy of grading was validated in randomly selected biopsies by a consortium of 8 European LeukemiaNET hematopathologists. BM fibrosis grade in 192 pts with PMF treated with BAT for 24 (97 pts), 48 (63 pts), 54 (17 pts), 60 (9 pts), or 66 (6 pts) mo was determined from prospectively collected biopsies from an independent, multicenter, observational database. The majority of BAT patients were treated with hydroxyurea [HU], various sequential therapies, or watchful waiting; few pts were treated with interferon-alpha. Biopsies were performed at protocol-defined intervals for RUX-treated pts or at the discretion of the treating physician in BAT-treated pts, mostly based on changes in a patient's clinical condition. Changes from baseline in BM fibrosis grade were categorized as improvement, stabilization, or worsening. Changes over time in the extent of collagen deposition and osteosclerosis and in BM cellularity were evaluated in RUX-treated pts only.
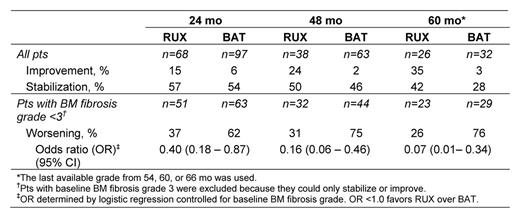
At baseline, 22%, 53%, and 25% of the 68 eligible RUX-treated pts had BM fibrosis grade 1, 2, and 3, respectively. Baseline osteosclerosis grade 0, 1, 2, and 3 was present in 50%, 32%, 9%, and 9% of RUX-treated pts, respectively. Accumulation of collagen fibers at baseline was observed in 32 pts (47%), with 29% having mild (grade 1) and 18% having manifest (grade 2) or intense (grade 3) collagen deposition. BM fibrosis grade distribution at baseline did not differ significantly between RUX- and BAT-treated pts (p=0.308, Cochran–Mantel–Haenszel test). At 24, 48, and 60 mo, a greater percentage of RUX-treated pts experienced stabilization or improvement of BM fibrosis relative to their baseline status compared with BAT-treated pts. Worsening BM fibrosis was more prevalent in BAT-treated pts compared with RUX-treated pts (Table). The majority of RUX-treated pts experienced durable spleen size reductions; effect on spleen size was associated with improvement or stabilization of BM morphology. In RUX-treated pts, improvement or stabilization of BM fibrosis at 24 mo was associated with a reduced relative risk of death compared with worsening BM fibrosis at 24 mo.
Long-term therapy with RUX may provide a clinically meaningful delay of BM fibrosis progression in pts with MF. The lack of a beneficial effect of long-term BAT is consistent with previous observations in a smaller cohort of HU-treated pts (Kvasnicka et al. ASCO 2013. Abstract 7030). Improvement or stabilization of BM fibrosis with RUX may be associated with more favorable outcomes. Results from this analysis support the importance of BM fibrosis as a prognostic marker in pts with MF.
Kvasnicka:Novartis: Consultancy; Incyte Corporation: Consultancy; Novartis: Research Funding; Incyte Corporation: Research Funding; Shire: Research Funding; AOP Orphan Pharmaceuticals: Research Funding; Novartis: Honoraria; Shire: Honoraria; Incyte Corporation: Honoraria. Thiele:AOP Orphan Pharmaceuticals: Consultancy; Incyte Corporation: Consultancy; Novartis: Consultancy; Shire: Consultancy; Sanofi: Consultancy; Novartis: Research Funding; Shire: Research Funding; AOP Orphan Pharmaceuticals: Honoraria; Incyte Corporation: Honoraria; Novartis: Honoraria; Shire: Honoraria; Sanofi: Honoraria. Sun:Incyte: Employment; Incyte: Equity Ownership. Cortes:Incyte, Sanofi: Consultancy; Incyte, Sanofi: Research Funding. Kantarjian:Ariad: Research Funding; Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding. Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal