Abstract
The impact of JAK inhibitor (JAKi) therapy on the natural history of myelofibrosis remains unclear. While a Phase 3 study showed that overall survival of ruxolitinib-treated intermediate-2 or high-risk myelofibrosis patients was superior as compared to placebo-treated patients, it remains unclear whether such an advantage would persist in patients receiving standard therapy, and whether this result is generalizable to other JAK inhibitors. We conducted a sponsor-independent, single-center retrospective analysis of overall and leukemia-free survival after long-term follow up in patients receiving JAKi treatment with comparison to two control populations: (i) a contemporaneously diagnosed (2005 onwards) primary myelofibrosis (PMF) patient cohort receiving standard therapy (JAKi and pomalidomide naïve); and (ii) a pomalidomide treated patient cohort (JAKi-naïve).
Baseline clinical, cytogenetic and molecular data were collected at the onset of study treatment with the first JAKi or pomalidomide, or at the time of first referral for patients receiving standard therapy. Information regarding bone marrow karyotype and DIPSS-plus risk status was available for all patients. Information on survival and leukemic transformation was updated in July 2013.
The 3 patient groups were: (i) JAKi-treated: n=157, median age 65 years, range 34-89. DIPSS-plus risk: Low/Int-1 20 (13%), Int-2 66 (42%), and High 71 (45%). Median follow up from first JAKi treatment was 30 months (range 1-67 months); during this period, 72 deaths (46%) and 14 (9%) leukemic transformations were documented. The first JAKi was ruxolitinib in 51 patients (33%), momelotinib (CYT387) in 79 (50%), and fedratinib (SAR302503) in 27 (17%). The proportion of patient deaths and leukemic transformation (median follow up, range) per specific JAKi was: ruxolitinib 57% and 12% (40 months, 2-67), momelotinib 43% and 9% (29 months, 1-42), and fedratinib 33% and 4% (21 months, 2-53), respectively.
(ii) Control#1 (Pomalidomide-treated): n=69, median age 67 years, range 36-87. DIPSS-plus risk: Low/Int-1 0 (0%), Int-2 28 (41%), and High 41 (59%). Median follow up was 25 months (range 2-70 months); during this period, 47 deaths (68%) and 7 (10%) leukemic transformations were documented.
(iii) Control# 2 (Standard therapy): n=319, median age 66 years, range 22-90. DIPSS-plus risk: Low/Int-1 98 (31%), Int-2 122 (38%), and High 99 (31%). Median follow up from time of referral was 18 months (range 1-92 months); during this period, 101 deaths (32%) and 18 (6%) leukemic transformations were documented. For patients with Int-2 or high-risk disease (n=221), 92 deaths (42%) and 13 (6%) leukemic transformations were recorded (median follow up 16 months, range 1-92).
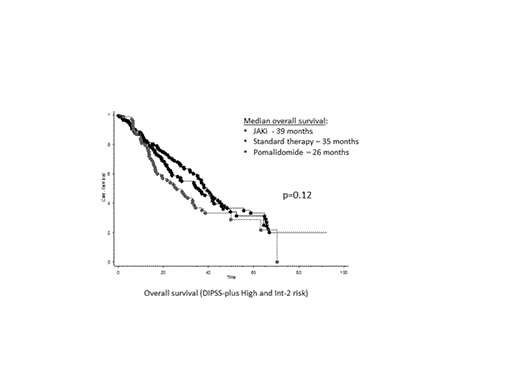
The survival analysis focused on DIPSS-plus Int-2 and high-risk patients for all groups, reflecting the population predominantly selected for treatment on JAKi clinical trials. There was no significant difference in the median overall survival of myelofibrosis patients treated with JAKi (39 months) versus standard therapy (35 months) versus pomalidomide (26 months) (p=0.12) (Figure). The 5-year survival rates were 31%, 33% and 26%, respectively. Similarly, the median overall survival of patients treated with ruxolitinib (35 months), momelotinib (37 months) or fedratinib (42 months) was not significantly different (p=0.4). Baseline DIPSS-plus status (ie, Int-2 vs. high-risk) effectively stratified JAKi treated patients in terms of overall survival (median survival 31 vs. 60 months; p=0.0002).
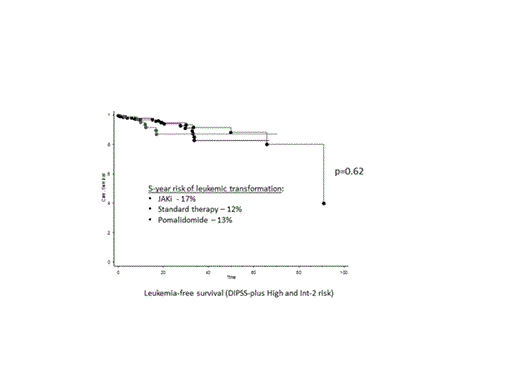
There was no significant difference in leukemia-free survival when comparing JAKi-treated patients versus standard therapy verus pomalidomide-treated patients (p=0.6) (Figure). The estimated 5-year risk of leukemic transformation was 17%, 12% and 13%, respectively.
With long-term follow up, myelofibrosis patients receiving JAKi therapy exhibited similar overall and leukemia-free survival as compared to two control populations (DIPSS-plus matched standard therapy and pomalidomide-treated patients). Baseline DIPSS-plus status was a powerful predictor of overall survival in JAKi treated patients. While underscoring the limitation of a non-head-to-head retrospective comparison, there was no significant difference in overall or leukemia-free survival when comparing individual JAKi drugs.
Pardanani:Bristol Myers Squibb: Clinical trial support Other; Sanofi: Clinical trial support, Publication support services, Clinical trial support, Publication support services Other; PharmaMar: Clinical trial support, Clinical trial support Other; JW Pharmaceutical Corp.: Clinical trial support, Clinical trial support Other. Off Label Use: Use of Ruxolitinib, Momelotinib, Fedratinib and Pomalidomide for treatment of Myelofibrosis in Clinical Trial setting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal