Abstract
A potential mechanism of resistance to FLT3 kinase inhibitors is high levels of FLT3 ligand (FL) which is commonly seen after treatment with myelosuppressive chemotherapy. We hypothesized that combining sorafenib with a less myelosuppressive agent, such as 5-azacytidine (AZA), may lead to higher and more durable responses. Furthermore, differentiation of leukemic blasts has been reported with both agents.
Patients were eligible if they had relapsed or refractory AML, were 18 years of age or older, and had adequate performance status (ECOG 2 2) and organ function. Patients older than 60 without prior therapy were also eligible if they were unsuitable to receive chemotherapy or refused it. Presence of FLT3-ITD was not a requirement but these patients were sought for participation in the study. Treatment regimen included AZA 75 mg/m2 daily for 7 days together with sorafenib 400 mg twice daily for 28 days; cycles were repeated in approximately 4 to 5-week intervals. Dose adjustments of both agents, and delay of AZA, based on toxicity were allowed. Plasma samples were collected on Day 1 and Day 10 of each cycle.
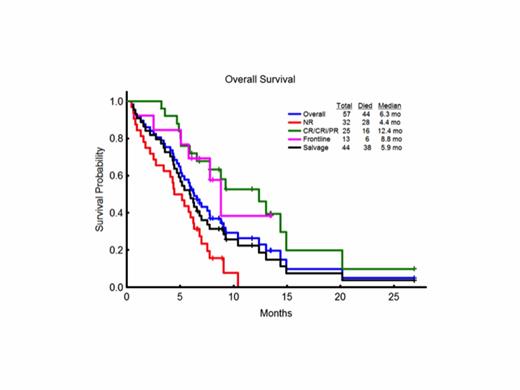
Overall, 57 patients with AML with a median age of 65 years (range, 21-87) were enrolled. They included 27 (47%) patients with normal cytogenetics, 12 (21%) with chromosome 5/7 or complex cytogenetic abnormalities, 15 (26%) with other miscellaneous abnormalities; 3 (5%) had insufficient metaphases. Prior to the initiation of treatment, FLT3-ITD was detected in 53/57 (93%) patients with a median allelic ratio of 0.348 (range, 0.009 – 0.934). They had received a median of 2 prior treatments (range, 0 -7) including 13 previously untreated patients. Twenty (35%) patients had received ≥3 prior regimens and 12 had failed prior therapy with FLT3 kinase inhibitors (6 with AC220, 2 with PKC412, and 9 with sorafenib, either as monotherapy or with chemotherapy or plerixafor); 4 patients had failed 2 prior FLT3 inhibitors. The overall CR/CRi/PR rate among the 57 patients is 44%, including 16 (28%) with CRi and 8 (14%) with CR and 1 (2%) with PR. The response rate among the previously untreated patients was 62% and among relapsed patients 39%. Patients have received a median of 3 (range, 1 - 27) treatment cycles with the median number of cycles to response among the responders being 2 (range, 1 – 4) and the median time to achieving response, 2.1 months (range, 0.8 – 4.6 months). The median duration of CR/CRi Is 2.4 months (range, 0.8 – 24.8 months). Seven patients have proceeded to allogeneic stem cell transplant. The most common study drug-related adverse events were rash and fatigue with no deaths attributable to study medications. One patient developed grade 3 cardiomyopathy. With a median follow-up of 8.6 months (range, 6.1 – 26.9), 13 patients remain alive, 5 still in remission. The median overall survival of the 57 patients was 6.3 months, and 12.4 months in the 25 responding patients. Mean FL levels at cycle 2, day 0 and cycle 2, day 10 were 27 pg/mL and 54 pg/mL, respectively, which is significantly lower than those seen previously in studies of FLT3 kinase inhibitor plus chemotherapy.
Combination of AZA and Sorafenib is effective for the treatment of patients with AML and FLT3-ITD. FL levels are significantly lower during the course of therapy with this combination compared to previously reported levels after chemotherapy.
Ravandi: Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal