Abstract
Glucocorticoids (GCs) such as prednisolone and dexamethasone are critical components of multi-agent chemotherapy regimens used in the treatment of acute lymphoblastic leukemia (ALL). Children with ALL are stratified into risk groups based on diagnostic features (i.e. age and cytogenetics) and therapy response. It has been established that the initial response to prednisolone is a major prognostic factor. Moreover, at relapse, de novo or acquired resistance to GCs is common and represents an important determinant in treatment failure. Recent studies performed by us and others have identified IKZF1 gene deletions and mutations as an independent prognostic factor that predicts prognosis and treatment outcome of children with B cell precursor ALL (BCP-ALL). These monoallelic IKZF1 gene deletions either affect the whole gene or may result in expression of dominant-negative IKZF1 isoforms due to intragenic deletions. However, it has not been established whether loss of IKZF1 function directly impacts the response to glucocorticoids.
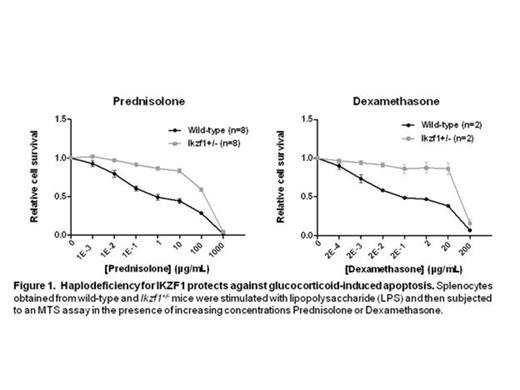
We examined whether haplodeficiency for Ikzf1 gene expression in mouse lymphocytes affects glucocorticoid-induced apoptosis. Splenocytes from Ikzf1+/- knockout mice were activated with lipopolysaccharide (LPS) and treated with increasing concentrations of either prednisolone or dexamethasone for 48 hours. B-lymphocytes haplodeficient for IKZF1 showed a significantly enhanced survival after treatment with GCs compared to wild type cells, as measured in an MTS assay and by AnnexinV staining. In case of prednisolone, the inhibitory concentration (IC50) was about ∼200-fold higher in the Ikzf1+/- splenocytes as compared to the wild-type cells. Gene expression analysis revealed that Ikzf1+/- splenocytes displayed lower overall expression levels as well as diminished transcriptional activation of several glucocorticoid receptor (GR)-induced target genes (i.e. Sgk1, Irs2, Zfp36L2). Furthermore, in luciferase reporter assays we established that IKZF1 overexpression enhances GR-mediated transcriptional activation in response to prednisolone. Finally, lentivirus-mediated IKZF1-shRNA expression in Nalm6 cell line, which reduces endogenous IKZF1 protein levels to around 50%, inhibits prednisolone and dexamethasone-induced apoptosis, demonstrating that also in human leukemia cells reduced IKZF1 expression levels protect against GC-induced cell death.
In conclusion, our data provide evidence that loss of IKZF1 function mediates resistance to glucocorticoid-induced apoptosis, which may contribute to the poor outcome of IKZF1-deleted BCP-ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal