Abstract
ONC201 (TIC10) is a novel small molecule that induces TRAIL-dependent apoptosis in various cancer cell types and is under development to enter a first-in-man study in advanced cancer patients .It was identified in a screen for small molecules capable of up-regulating endogenous TRAIL gene transcription in a p53-independent manner (Allen JE et al, Sci Transl Med., 2013). ONC201 triggers FOXO3a activation through dual inhibition of ERK and AKT, which transcriptionally upregulates TRAIL and TNFRSF10B (TRAIL-R2/DR5) in solid tumors. Because PI3K/AKT and MEK/ERK activation have been shown to be major contributors to drug resistance, ONC201 is potentially promising since it not only promotes TRAIL activation, but also upregulates its pro-apoptotic receptor DR5.
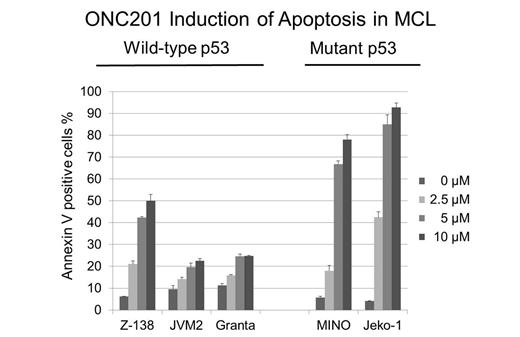
To determine the significance of p53 functional status in ONC201-induced apoptosis, p53 wild-type Z-138 and JVM-2 cells were stably transduced with lentivirus encoding either negative control shRNA or p53-specific shRNA and were exposed to ONC201 and results demonstrated complete p53-independence. Normal human bone marrow cells and mesenchymal stem cells were completely resistant to the cytotoxic effects of ONC201, which illustrated this agent's low toxicity against normal tissues.
In order to examine the role of p53 activation in ONC201-induced apoptosis in MCL cells, we combined ONC201 with the MDM2 inhibitor Nutlin-3a. The combination cytotoxic effects of this combination were synergistic in p53 wild-type Z-138 and JVM-2 cells (combination index 0.87 and 0.63, respectively). Similar synergistic effects of ONC201 combined with the BTK inhibitor Ibrutinib were observed in Z-138 and MINO cells (combination index 0.63 and 0.61, respectively). This combination also triggered synergistic apoptotic effects in two primary MCL samples with combination indexes of 0.0011 and 0.073, respectively.
ONC201 induces p53-independent apoptosis in MCL cells, and may have significant clinical impact by targeting both p53 wild type and p53 mutant drug-resistant MCL cells. ONC201 exerts synergistic effects with MDM2 and BTK inhibitors that may be explored clinically.
Allen:Drug Company: Employment. Andreeff:Oncoceutics: SAB Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal