Abstract
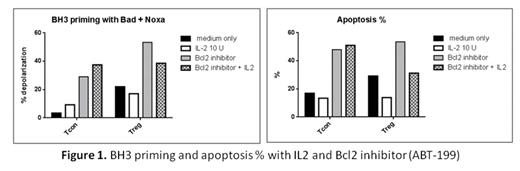
CD4+CD25+Foxp3+ regulatory T cells (Treg) play an important role in the maintenance of self-tolerance and immune homeostasis. Interleukin-2 (IL-2) is critical for Treg development, expansion, activity and survival. Lack of either IL-2 or IL-2Rα (CD25) results in Treg deficiency and Treg impairment is associated with loss of tolerance, autoimmunity, and chronic GVHD. We previously demonstrated that daily administration of low-dose IL-2 resulted in selective expansion of Treg in vivo and clinical improvement of chronic GVHD (Koreth et al. NEJM 2011). We also reported that low-dose IL-2 enhanced Treg resistance to FAS-mediated apoptosis through the extrinsic pathway (Matsuoka et al. Sci Transl Med 2013). However susceptibility of Treg to apoptosis through the intrinsic pathway has not been examined. To examine the mechanisms whereby IL-2 affects susceptibility of Treg to apoptosis through the intrinsic pathway, we used a functional flow cytometry-based assay (BH3 profiling) to measure mitochondrial membrane depolarization in response to a panel of pro-apoptotic BH3 peptides (BIM, BID, BAD, NOXA, PUMA, BMF, HRK). Mitochondria that are more sensitive to the BH3 peptides are more 'primed' for apoptosis, and more sensitive to pro-apoptotic signaling. This assay allowed us to compare “priming” which we define as susceptibility to BH3 peptide-induced mitochondrial membrane depolarization in Treg and Tcon. We also examined cytoplasmic expression of Bcl-2, BclxL, Mcl1, Bim, XIAP, Ki67 and Staurosporine (STS) induced apoptosis as additional measures of susceptibility to apoptosis and proliferation in each subset. In resting blood obtained from healthy donors (n=25), Treg were more “primed” than Tcon when exposed to several BH3 peptides (Bim, Bad, PUMA and BMF). This BH3 peptide pattern suggests that the anti-apoptotic protein, Bcl2 plays a primary role in the priming of these cells. Analysis of pro- and anti-apoptotic proteins revealed that Treg expressed higher levels Bim and BclxL and lower levels of Bcl2 and XIAP than Tcon, but there was no significant difference in Mcl1. Treg expressed higher levels of Ki67 and were more susceptible to both STS- and FAS-induced apoptosis than Tcon. Thus, Treg in healthy individuals have higher proliferative activity and are more susceptible to apoptosis than Tcon through both mitochondrial and death receptor pathways.
Letai:AbbVie: Consultancy; Dana-Farber Cancer Institute: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal