Abstract
Chromosomal analysis using conventional cytogenetics (CC) and interphase fluorescence in situ hybridization (FISH) identifies a high risk multiple myeloma population (HR-MM) characterized by poor response to chemotherapy and shorter survival. The objective of this study was to compare outcomes of autologous hematopoietic stem cell transplantation (ASCT) at our institution in HR-MM and standard risk MM (SR-MM) patients (pts) identified through CC and FISH.
Metaphase CC and FISH data was collected from medical records of MM pts who underwent ASCT at our institution between Jan 2005 to Dec 2009. HR-MM pts were defined if CC revealed deletion (del) of chromosome 13/13q, del17/17p, t(4;14), t(14;16), hypoploidy (<45 chromosomes excluding –Y), or chromosome 1 abnormalities [+1, -1, t(1;x)] in at least 2 metaphases; or if FISH showed del 17p, t(4;14). Kaplan & Meier method was used for assessing progression free survival (PFS) and overall survival (OS); log-rank test was used to evaluate the difference between pt groups and Cox proportional hazards models were fitted for multivariate analysis.
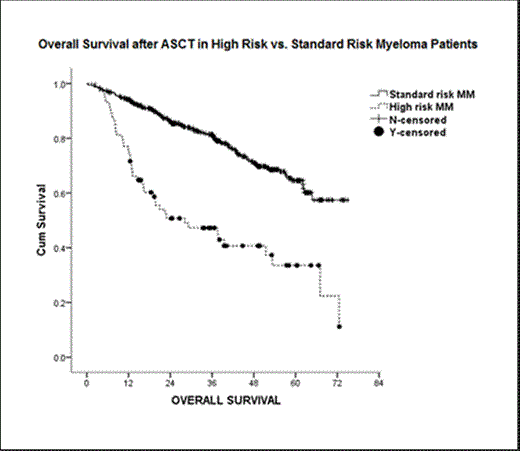
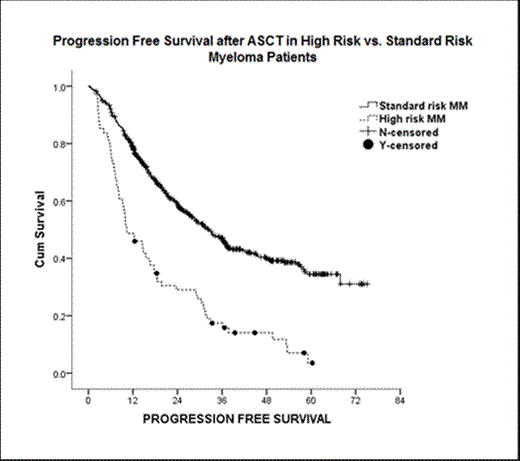
Out of 670 MM pts who received ASCT during the specified time, 74 (11%) had HR-MM while 596 (89%) had SR-MM. Table 1 summarizes baseline characteristics and outcomes of HR- and SR-MM pts. In HR-MM pts, chromosome 1 aberrations were most common and seen in 53 (76%) pts while del13/13q, hypodiploidy, del17/17p and t(4;14) was found in 48 (65%), 27 (37%), 16 (22%) and 5 (9%) pts, respectively. Concurrence of high-risk chromosomal abnormalities (i.e. >1) occurred in 58% of HR-MM pts. As compared to SR-MM pts, HR-MM pts were more commonly male (72 vs. 56%), had a significantly higher bone marrow plasma cell burden at diagnosis (52 vs. 29%. p<0.001) or at time of ASCT (5 vs. 2%. p<0.000) and were refractory to treatment before undergoing ASCT (34 vs. 22%; p0.01). Melphalan 200mg/m2 was the most common conditioning regimen used and there was no difference in time to bone marrow engraftment and platelet recovery. With median follow up of 39.8 months after ASCT, HR-MM pts, as compared to SR-MM pts, had significantly lower rates of >/= PR (74 vs. 84%; p <0.01), median PFS (10.3 vs. 32.4 months p<0.001) and OS (28 vs. not reached, p<0.001). On multivariate analysis of the HR-MM pts, having only 1-high-risk cytogenetic abnormality or achieving at least very-good-partial-response (VGPR) after ASCT were factors independently associated with improved OS (p<0.002). In contrast, induction chemotherapy with bortezomib, myeloma status at time of ASCT and post-ASCT maintenance chemotherapy did not significantly affect PFS or OS.
Patient characteristics and outcomes
| Patient . | High risk MM . | Standard risk MM . | p value . |
|---|---|---|---|
| N (%) | N (%) | ||
| Median (range) | Median (range) | ||
| Age | 74 (11) | 596 (89) | |
| Gender | 58 (40-73) | 58 (31-80) | 0.88 |
| Male | 53 (72) | 335 (56) | 0.003 |
| Race | 52 (71) | 407 (66) | 0.61 |
| White | 10 (13) | 103 (17) | |
| Black | 12 (16) | 103 (17) | |
| Other | |||
| Histology | 26 (49) | 339 (59) | 0.03 |
| IgG | 14 (26) | 123 (21) | |
| IgA | 6 (11) | 96 (16) | |
| Light chain only | 8 (11) | 21 (4) | |
| Other | |||
| Durie Salmon stage | 5 (7) | 111 (19) | 0.03 |
| I | 35 (49) | 237 (42) | |
| II | 32 (44) | 225 (39) | |
| III | |||
| Bone marrow plasma cell % | 52 (0-95) | 29 (0-98) | <0.000 |
| At diagnosis | 5 (0-92) | 2 (0-88) | <0.000 |
| Before ASCT | |||
| Cytogenetic and FISH abnormalities | 53 (72) | N/A | |
| Chromosome 1 | 48 (65) | ||
| del13/13q | 16 (22) | ||
| del17/17p | 5 (9) | ||
| t(4;14) | 27 (37) | ||
| hypodiploidy | |||
| Concurrence of HR abnormalities | 31 (42) | N/A | |
| 1 | 43 (58) | ||
| 1 | |||
| At least partial response to pre ASCT induction chemotherapy | 56 (77) | 499 (85) | 0.06 |
| Disease status at the time of ASCT | 48 (66) | 461 (78) | 0.01 |
| First remission | 25 (34) | 127 (22) | |
| Relapsed/Refractory disease | |||
| Interval to ASCT (months) | 7 (2-159) | 7 (1-262) | 0.84 |
| Conditioning regimen | 43 (81) | 500 (81) | 0.98 |
| Melphalan 200mg/m2 | |||
| Engraftment (days) | 10 (9-12) | 10 (0-20) | 0.57 |
| Neutrophil >0.5 x 109/L | 11 (0-20) | 11 (0-70) | 0.42 |
| Platelet >20 x 109/L | |||
| Response to ASCT | 5 (7) | 70 (12) | 0.000 |
| sCR | 6 (8) | 110 (19) | |
| CR | 18 (24) | 163 (28) | |
| VGPR | 26 (35) | 154 (26) | |
| PR | 8 (11) | 72 (12) | |
| SD | 11 (15) | 22 (4) | |
| PD |
| Patient . | High risk MM . | Standard risk MM . | p value . |
|---|---|---|---|
| N (%) | N (%) | ||
| Median (range) | Median (range) | ||
| Age | 74 (11) | 596 (89) | |
| Gender | 58 (40-73) | 58 (31-80) | 0.88 |
| Male | 53 (72) | 335 (56) | 0.003 |
| Race | 52 (71) | 407 (66) | 0.61 |
| White | 10 (13) | 103 (17) | |
| Black | 12 (16) | 103 (17) | |
| Other | |||
| Histology | 26 (49) | 339 (59) | 0.03 |
| IgG | 14 (26) | 123 (21) | |
| IgA | 6 (11) | 96 (16) | |
| Light chain only | 8 (11) | 21 (4) | |
| Other | |||
| Durie Salmon stage | 5 (7) | 111 (19) | 0.03 |
| I | 35 (49) | 237 (42) | |
| II | 32 (44) | 225 (39) | |
| III | |||
| Bone marrow plasma cell % | 52 (0-95) | 29 (0-98) | <0.000 |
| At diagnosis | 5 (0-92) | 2 (0-88) | <0.000 |
| Before ASCT | |||
| Cytogenetic and FISH abnormalities | 53 (72) | N/A | |
| Chromosome 1 | 48 (65) | ||
| del13/13q | 16 (22) | ||
| del17/17p | 5 (9) | ||
| t(4;14) | 27 (37) | ||
| hypodiploidy | |||
| Concurrence of HR abnormalities | 31 (42) | N/A | |
| 1 | 43 (58) | ||
| 1 | |||
| At least partial response to pre ASCT induction chemotherapy | 56 (77) | 499 (85) | 0.06 |
| Disease status at the time of ASCT | 48 (66) | 461 (78) | 0.01 |
| First remission | 25 (34) | 127 (22) | |
| Relapsed/Refractory disease | |||
| Interval to ASCT (months) | 7 (2-159) | 7 (1-262) | 0.84 |
| Conditioning regimen | 43 (81) | 500 (81) | 0.98 |
| Melphalan 200mg/m2 | |||
| Engraftment (days) | 10 (9-12) | 10 (0-20) | 0.57 |
| Neutrophil >0.5 x 109/L | 11 (0-20) | 11 (0-70) | 0.42 |
| Platelet >20 x 109/L | |||
| Response to ASCT | 5 (7) | 70 (12) | 0.000 |
| sCR | 6 (8) | 110 (19) | |
| CR | 18 (24) | 163 (28) | |
| VGPR | 26 (35) | 154 (26) | |
| PR | 8 (11) | 72 (12) | |
| SD | 11 (15) | 22 (4) | |
| PD |
HR-MM pts are characterized by higher bone marrow plasma cell burden, concurrence of high-risk chromosomal abnormalities, and poor response to induction or high dose chemotherapy followed by ASCT as compared to SR-MM pts. In HR-MM pts, having only 1-high risk cytogenetic abnormality or achieving at least VGPR with ASCT are independent factors associated with improved survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal