Abstract
Although treatment with purine analogs is associated with a high response rate, hairy cell leukemia (HCL) remains incurable with a relapse rate of approximately 30%. For these patients (pts), novel therapies are needed. The recent major finding of the near universal and exclusive presence of BRAFV600E mutations in HCL has identified BRAF as a promising therapeutic target. BRAF inhibition in HCL may not only represent the first targeted therapeutic approach, but also a more effective strategy for treatment of HCL. Therefore, we have designed a phase II trial to determine the clinical efficacy of the BRAF inhibitor vemurafenib in pts with relapsed or refractory HCL.
Pts with HCL who are resistant to/intolerant of purine analogs, or who have achieved suboptimal response to purine analogs (i.e. relapse 22 years following completion of therapy, or who have ≥ 2 relapses) were enrolled to the trial. Pts must have an indication for treatment, as defined by ANC 21.0, HGB 210, PLT 2100K. Eligible pts received vemurafenib 960mg bid continuously in cycles of 4 weeks for 3 cycles. Bone marrow (BM) evaluations were performed after cycle 1 and 3. Pts with partial response (PR) or complete response (CR) with detectable minimal residual disease (MRD) were allowed to receive vemurafenib for up to 3 additional cycles at the discretion of treating physicians. Serial peripheral blood (PB) and/or BM samples were tested for BRAF IHC, quantitative BRAF mutant allele burden assessment by allele-specific qRT-PCR, serum cytokine analysis, detailed multiparameter flow cytometric analysis to identify the effect of vemurafenib on myeloid and lymphoid cell regeneration, and targeted next-generation sequencing analysis of a panel of 200 genes known to be mutated in cancer and/or associated with response to MAP kinase pathway inhibitors to detect potential predictors of response to vemurafenib and identify genes collaborating with BRAF mutations in HCL pathogenesis.
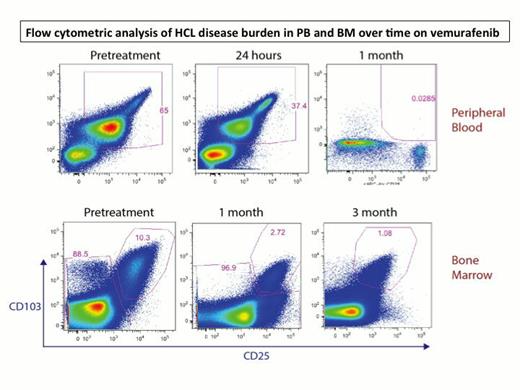
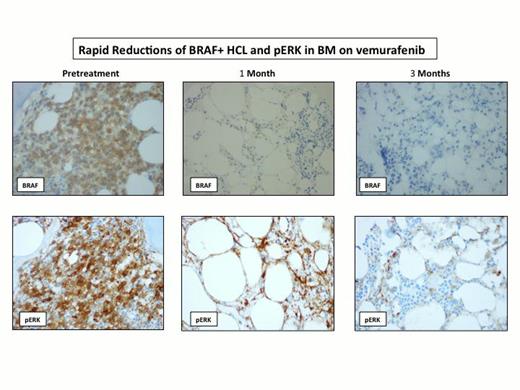
To date, 9 pts have been enrolled and 8 pts received the treatment. The median age was 62 years (range 49-77 years), and the median number of treatments prior to the study was 3.5 (range 1-7), including 2 pts with splenectomy. 5 pts are evaluable for toxicity and disease response. The most common adverse events were rash (Grade 2: 4 pts), photosensitivity (Grade 1-3: 3 pts), arthralgia (Grade 2-3: 3 pts), hand-foot syndrome (Grade 2: 1 pt), febrile neutropenia (Grade 3: 1 pt) and tumor lysis syndrome (Grade 3: 1 pt). One pt developed new squamous cell carcinoma while on therapy and successfully underwent complete resection. 4 pts required dose reductions to 480mg bid due to side effects of arthralgia, symptomatic hand-foot syndrome and febrile neutropenia, but all were able to complete all intended treatment. All 5 pts achieved complete hematologic recovery. 2 pts achieved marrow CR (1 MRD positive and the other MRD negative), and 3 pts achieved marrow PR with very minimal disease. Responses were rapid and evident by PB flow cytometric analysis 24 hours after the initial dose (Figure 1). Plasma levels of sCD25 normalized within 2-3 weeks, and this correlated with a rapid decrease of BRAF+ hairy cells and dephosphorylation of ERK after one cycle (Figure 2). Several additional inflammatory cytokines correlated closely with the burden of leukemic cells identifying novel tumor markers in HCL including sTNF-R2, sIL-1R2, and sIL4R. Continued improvement in response rates was noted when assessed at the end of cycle 1 and 3, as documented by paired PB and BM multiparametric flow cytometric analysis. 2 pts with PR after cycle 1 converted to CR after cycle 3, and, in the remaining 3 pts who maintained PR, the amount of disease burden further decreased from cycle 1 to cycle 3. Genetic analysis is ongoing and has identified mutations in MLL2 and CREBBP, genes previously described to be mutated in other B cell malignancies but not previously described in HCL.
While longer follow-up is needed to assess the durability of the response, our results indicate that vemurafenib has potent antitumor activity in pts with relapsed/refractory BRAF mutant HCL and confirm the MAP kinase pathway as a rational promising therapeutic target in HCL. However, the optimal dose and duration of the drug in HCL remain unknown, and further studies are warranted. Enrollment is ongoing to this study, and updated clinical and genetic analysis data will be presented.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal