Abstract
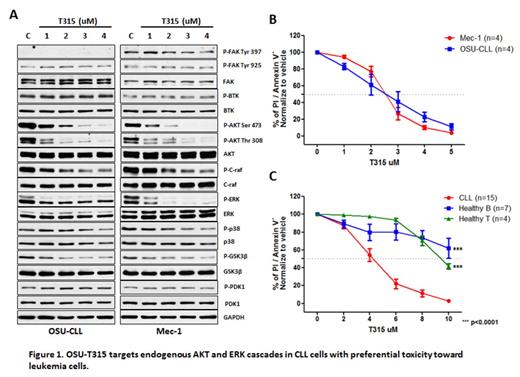
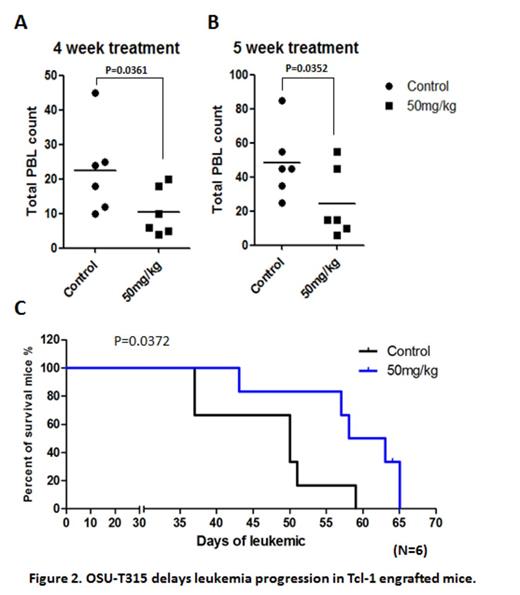
Aberrant regulation of endogenous survival pathways plays a major role in pathogenesis of chronic lymphocytic leukemia (CLL). Signaling via conjugation of surface receptors within the tumor environmental niche is believed to activate survival and proliferation pathway in CLL. PI3K/AKT appears to be the pivotal axis to support CLL growth. Current studies identify Integrin-linked kinase (ILK) as a Phosphoinositide-dependent kinase-2 (PDK2), by which it mediates AKT activation and concomitant with tumorigenesis in many solid tumors. As a result of our search for novel therapeutic targets, we first report the potent inhibitor, OSU-T315, modeled from the scaffold that docks into AKT-binding site of ILK in therapeutic of hematological malignancy. OSU-T315 triggers apoptosis in CLL by targeting both intrinsic and extrinsic AKT and ERK activation (Figure 1A). OSU-T315 induces cytotoxicity to both CLL-derived cell lines Mec-1 and OSU-CLL, as well as in primary CLL cells, while illustrating alleviated toxicity in normal B and T cells (Figure 1B, C). In contrast to the highly successful BTK and PI3 kinase inhibitors which inhibit BCR signaling proximally, OSU-T315 specifically inhibits down-stream AKT and ERK signaling cascades without affecting proximal BCR or focal-adhesion signaling. In particular, external stimuli through BCR or CD49d, by which triggers survival signal and protects CLL from ankoisis via AKT/ERK activation, is potently inhibited by OSU-T315. Downstream anti-apoptotic molecule Mcl-1 is suppressed upon OSU-T315 treatment, which is later accompanied by increased Caspase 3/7 activity. Further study indicates that OSU-T315 mediated cell death is Caspase dependent. In vivo efficacy of OSU-T315 is supported by a therapeutic study in the TCL1 mouse model of CLL, from which demonstrates improvement in total counts of white blood cells and reduced splenomegaly, thereby contributing to enhanced overall survival following OSU-T315 oral treatment regimen (Figure 2). Together, our findings suggest the innovative dual-targeted approaches by suppressing both AKT and ERK pathway in CLL therapy through inhibiting ILK, and provide an alternative agent for potential therapeutic development in CLL.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal