Abstract
Most patients with acute myeloid leukemia (AML) become resistant to chemotherapy at some point in their course and succumb to their disease. It is necessary to prevent chemo-resistance or enhance chemosensitivity in a selective fashion to lead to a higher cure rate and a lower toxic burden. The B-cell leukemia /lymphoma 2 (BCL-2) protein acts to prevent commitment to programmed cell death initiated in the mitochondrion. Inhibiting the function of this protein is an attractive approach to cancer therapy, but it remains a challenge to identify those tumors that will best be treated with such a strategy. Here, we test the sensitivity of AML cell lines and primary patient samples to the selective BCL-2 inhibitor, ABT-199, and test for correlation between ABT-199 sensitivity with BCL-2 dependence measured by BH3 profiling. We hypothesized that cells that are more dependent on BCL-2 will be more sensitive to inhibition by ABT-199.
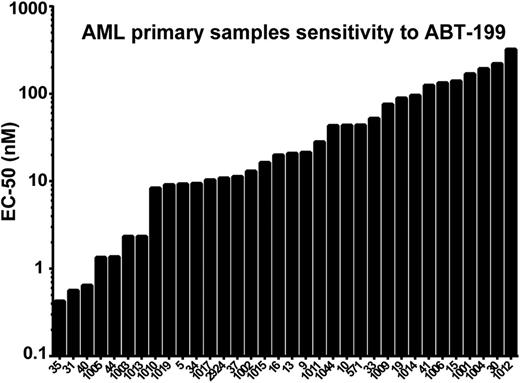
To this end, we tested the sensitivity of seven AML cell lines and 34 primary patient samples to ABT-199. We found that both cell lines and primary patient samples were sensitive to ABT-199 (Figure 1). In the case of primary patient cells, the median EC-50 was approximately 20 nM, and cell death could be seen within 2 hours. Importantly, we found that sensitivity to ABT-199 was independent of cytogenetics and NPM1 and FLT3 mutations of the primary patient cells suggesting that treatment with ABT-199 could be useful for a variety of AML patients, even those with an intrinsically poor prognosis.
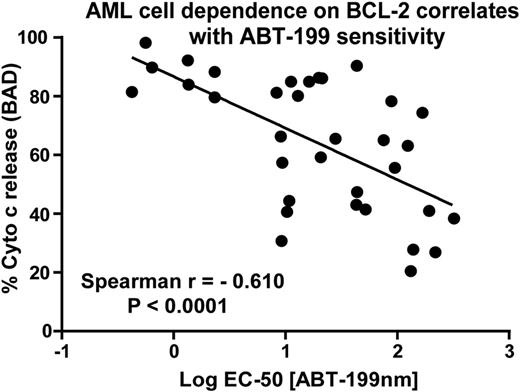
We also measured dependence on BCL-2-mediated apoptosis in cell lines and primary patient samples by BH3 profiling. BH3-profiling is a method to determine the mitochondrial priming level of a cell by exposing cellular mitochondria to standardized amounts of peptides derived from the BH3 domains of BH3-only proteins and determining the rate of cytochrome c release. BH3-profiling can also identify which anti-apoptotic species are critical in mediating cell death in a given cell type. For instance, the BAD BH3-only protein binds with high affinity to BCL-2, BCL-XL and BCL-W. Thus, release of cytochrome c following BAD peptide incubation suggests an anti-apoptotic dependency on BCL-2, BCL-XL or BCL-W. Therefore, we tested whether ABT-199 sensitivity correlates with functional dependence on BCL-2 in both cell lines and primary patient samples. We found that cells that released cytochrome c following incubation with the BAD peptide were more sensitive to ABT-199 treatment (Figure 2). This demonstrates that ABT-199 functions on target at the mitochondria since the ABT-199 mitochondrial activity correlates well with ABT-199 cytotoxicity data.
Since ABT-199 does not inhibit MCL-1, increased expression of MCL-1 could be a potential source of upfront resistance to BCL-2 inhibition. Therefore, we asked if there was a correlation between MCL-1 dependence and ABT-199 sensitivity. We observed a weak anti-correlation between mitochondrial sensitivity to ABT-199 and sensitivity to the MCL-1 selective peptide NOXA BH3. This suggests that there is a minor tendency for MCL-1 dependent mitochondria to be less sensitive to ABT-199.
Letai:AbbVie: Consultancy; Dana-Farber Cancer Institute: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal