Abstract
Exportin 1 (XPO1) also called CRM1, is a widely expressed nuclear export protein, transporting a variety of molecules including tumor suppressor proteins and cell cycle regulators. Targeted inhibition of XPO1 is a new strategy to restore multiple cell death pathways in various malignant diseases. SINEs are novel, orally available, small molecule Selective Inhibitors of Nuclear Export (SINE) that specifically bind to XPO1 and inhibit its function.
We used WST-1 cell proliferation assays, flow cytometry, and bioluminescence imaging to evaluate the efficacy of multiple SINEs to induce apoptosis alone and in combination with cytarabine (AraC) or doxorubicin in vitro in chemotherapy sensitive and resistant murine acute promyelocytic leukemia (APL) cells. This murine model of APL was previously generated by knocking in the human PML-RARa cDNA into the 5’ regulatory sequence of the cathepsin G locus (Westervelt et al. Blood, 2003). The abnormal co-expression of the myeloid surface antigen Gr1 and the early hematopoietic markers CD34 and CD117 identify leukemic blasts. These Gr1+CD34+CD117+ APL cells partially retain the ability to terminally differentiate toward mature granulocytes (mimicking more traditional AML models) and can be adoptively transferred to secondary recipients, which develop a rapidly fatal leukemia within 3 weeks after tumor inoculation. To assess the safety and efficacy of SINEs in vivo, we injected cryopreserved APL cells intravenously via the tail vein into unconditioned genetically compatible C57BL/6 recipients and treated leukemic and non-leukemic mice (n=15/cohort) with 15 mg/kg of the oral clinical staged SINE KPT-330 (currently in Phase 1 studies in patients with solid tumors and hematological malignancies) alone or in combination with 200 mg/kg cytarabine every other day for a total of 2 weeks. Peripheral blood was obtained weekly from mice for complete blood counts and flow cytometry to screen for development of APL.
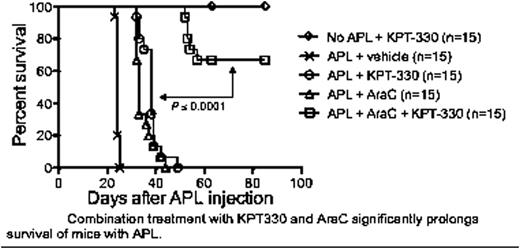
The first generation SINE, KPT214, inhibited the proliferation of murine APL cell lines in a dose and time dependent manner with IC50 values ranging from of 95 nM to 750 nM. IC50 values decreased 2.4-fold (KPT-185) and 3.5-fold (KPT-249) with subsequent generations of the SINEs. Consistent with the WST-1 results, Annexin V/7-aminoactinomycin D flow cytometry showed a significant increase of APL apoptosis within 6 hours of KPT-249 application. Minimal toxicity against normal murine lymphocytes was observed with SINEs even up to doses of 500 nM. Additional WST-1 assays using AraC-resistant and doxorubicin-resistant APL cell lines demonstrated cell death of both chemotherapy-resistant cell lines at levels comparable to the parental chemosensitive APL cell lines. Combination therapy with low dose KPT-330 and AraC showed additive effects on inhibition of cell proliferation in vitro. This additive effect of KPT-330 and chemotherapy on APL killing was maintained in vivo. As shown in Figure 1, treatment with AraC or KPT-330 alone significantly prolonged the survival of leukemic mice from a median survival of 24 days (APL + vehicle) to 33 days or 39 days, respectively (P < 0.0001). Encouragingly, combination therapy with AraC + KPT-330 further prolonged survival compared to monotherapy (P < 0.0001), with some mice being cured of the disease. Similar in vivo studies with the AraC-resistant and doxorubicin-resistant APL cells are just being initiated.
Combination treatment with KPT330 and AraC significantly prolongs survival of mice with APL.
Combination treatment with KPT330 and AraC significantly prolongs survival of mice with APL.
Our data suggests that the addition of a CRM1 inhibitor to a chemotherapy regimen offers a promising avenue for treatment of AML.
Shacham:Karyopharm Therapeutics Inc.: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Patents & Royalties. Kauffman:Karyopharm Therapeutics Inc.: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Patents & Royalties. McCauley:Karyopharm Therapeutics, Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal