Abstract
Protein disulfide isomerase (PDI) is a member of the group of sulfhydryl isomerase enzymes which catalyze the formation, reduction and exchange of disulfide bonds. We and others have shown that PDI has a role in integrin-mediated platelet adhesion and aggregation, and among its proposed targets are integrins αIIbβ3 and αvβ3. There has been no clear evidence indicating whether disulfide bond exchange plays a role in the post-ligation phase of adhesion, which involves outside-in signaling.
We tested our hypothesis that free sulfhydryls and PDI play an essential role in the post-ligation phase of αIIbβ3 and αvβ3-dependent cell adhesion to fibrinogen.
Baby Hamster Kidney (BHK) cells were transfected with wild type (WT) αIIb and either WT β3 or β3 mutated at specific cysteines, resulting in surface expression of both αvβ3 and αIIbβ3. Single or double cysteine to serine mutations disrupting the Cys473-Cys503 and the Cys523-Cys544 disulfide bonds were generated. Evaluation of the expression and activity of WT or mutated integrins on the surface of BHK cells was performed by flow cytometry using P2 and PAC1 antibodies, respectively. Adhesion of BHK cells, expressing WT or mutated αIIbβ3, to fibrinogen-coated wells was studied in the presence or absence of bacitracin, a PDI inhibitor. Each experiment was performed with and without an αvβ3blocker (RO0655233-001). The adhered cells were stained and counted using light microscopy.
Flow cytometry showed that BHK cells expressing the αIIbβ3 mutants bound both P2 and PAC1 while WT αIIbβ3-transfected cells bound only P2, indicating that the mutated αIIbβ3 receptors were constitutively active while WT αIIbβ3 was inactive as previously shown. The adhesion of BHK cells to fibrinogen was dependent on αIIbβ3 surface expression in both WT and mutants. Adhesion was reduced following treatment with the αvβ3 blocker, suggesting that both β3integrins support binding to immobilized fibrinogen (p < 0.05 for WT, C473S, C523S/C544S; p=0.053 for C544S).
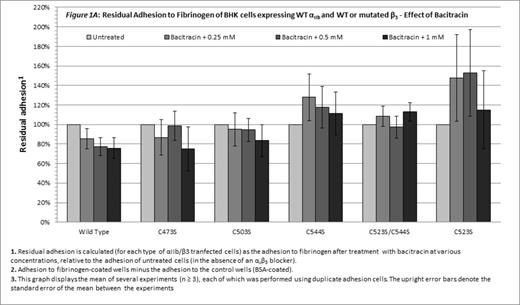
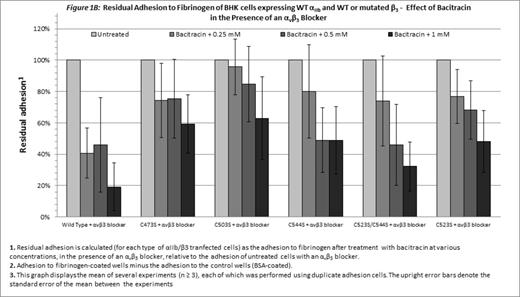
Without blocking of αvβ3, only cells expressing WT αIIbβ3 were inhibited by bacitracin (p=0.03, Figure 1A). Combination of the αvβ3 blocker and bacitracin, enabled a concentration-dependent inhibitory effect of bacitracin on adhesion of cells expressing either WT αIIbβ3or mutants disrupting the Cys523-Cys544 bond (C544S, C523S/C544S and C523S; p < 0.05 for each; Figure 1B). Statistical analysis failed to indicate a similar role for the Cys-473-Cys-503 bond (C473S and C503S; Figure 1B).
Cells expressing the constitutively active mutated αIIbβ3 were still dependent on PDI for adhesion, as shown by the inhibitory effect of the PDI inhibitor on their adhesion in the presence of an αvβ3 blocker, demonstrating that disulfide bond exchange plays an essential role in the post-ligation stage of αIIbβ3-mediated adhesion to fibrinogen. The difference between the inhibitory effect of bacitracin on the mutants disrupting the Cys523-Cys544 bond and the mutants disrupting the Cys473-Cys503 bond suggests that the role of disulfide bond exchange in the post-ligation phase of adhesion may differ between different disulfide bonds.
In the absence of the αvβ3 blocker, bacitracin had no inhibitory effect on cells expressing the constitutively active mutants and the inhibitory effect of bacitracin on cells expressing the WT integrins was less prominent. Therefore, disulfide bond exchange mediated by PDI may have a pivotal role in the post-ligation phase of adhesion mediated by αIIbβ3 receptor, while the adhesion to fibrinogen mediated by αvβ3integrin depends on PDI to a lesser extent.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal