Abstract
NK cells are innate lymphocytes that are important for host defense against infections, and are potent anti-cancer immune effectors. In peripheral blood, human NK cells are categorized into developmentally related, but functionally distinct, subsets. CD56dim NK cells, thought to be developmentally more mature, are the major subset in peripheral blood (80-95%), express perforin and granzyme B at rest and exhibit degranulation, cytotoxicity and IFN-γ responses against tumor targets without prior stimulation. In contrast, CD56bright NK cells, are less mature, are the major subset in secondary lymphoid tissues, lack expression of perforin and granzyme B and are associated with minimal degranulation, cytotoxicity, and IFN-γ responses to tumor targets. IL-15 has been shown to support the survival and proliferation of CD56bright NK cells, but its impact on the anti-leukemia response has not been reported. Here we investigate the impact of brief exposure to human recombinant IL-15 on functional responses of CD56bright and CD56dim NK cells to leukemia target cells including primary AML blasts.
Normal human donor NK cells (>95% purity) were cultured with cytokine free media (control) or with 5 ng/ml of rhIL-15 (primed) for 16 hours, washed and then tested for functional responses after co-incubation with K562 cells or primary AML blasts for 6 hours. NK cell functional responses assessed include degranulation, cytokine production and cytotoxicity (using flow based killing assays). For the tumor:nk cell conjugate analyses, pre-stained NK cells were co-incubated with CFSE labeled K562 cells and then CD56bright conjugate formation assessed by gating on CFSE+CD56+CD16- cells.
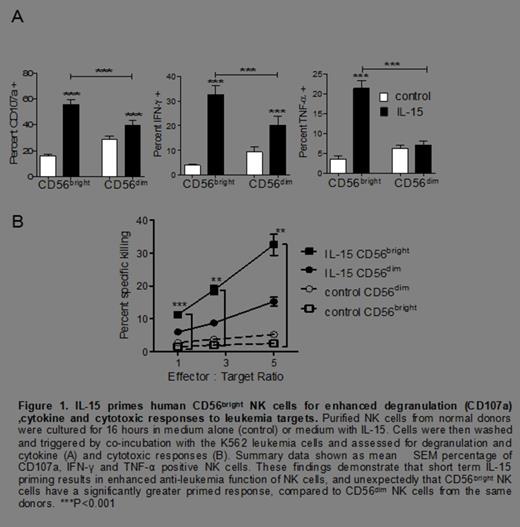
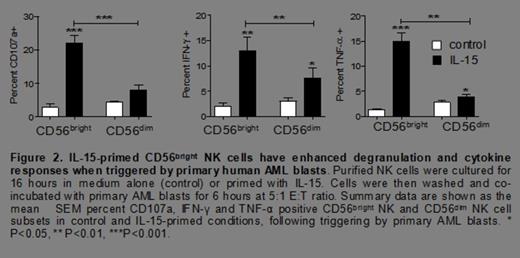
Anti-leukemia effector functions of human NK cells are classically attributed to the CD56dim subset, however after priming for 16 hours with rhIL-15 (5 ng/mL, a concentration that stimulates via the IL-2/15Rβγc), surprisingly, we observed that IL-15-primed CD56bright NK cells exhibited significantly greater degranulation (CD107a), cytokine (IFN-γ and TNF-α) and cytotoxic responses to both K562 leukemia cells (Figure 1) and to primary AML blasts (figure 2), compared to IL-15 primed CD56dim NK cells from the same donor. Further, we found a marked increase in the expression of perforin (70 ± 5% vs. 12 ± 6%, P< 0.0001), granzyme B (64 ± 5% vs. 12 ± 2.5%, P< 0.0001), and TRAIL (89 ± 2.5% vs. 6 ± 1%, P< 0.0001) in IL-15 primed CD56bright NK cells.
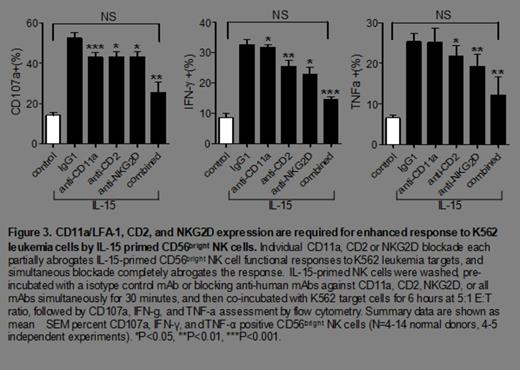
We found an increased number of tumor conjugates with the IL-15 primed, compared to control, CD56bright cells at 5 minutes (19 ± 3% vs. 3.5 ± 1%, P= 0.02), 15 minutes (22 ± 3% vs. 8 ± 2%, P= 0.0003) or at 30 minutes (13 ± 2% vs. 3.5 ± 1%, P= 0.008) from the same donors. Further, there was a significant increase in the expression of NKG2D (MFI of 8.5 ± 2 vs. 3 ± 0.5, P= 0.03), NKp30 (65 ± 4% vs. 21 ± 3%, P< 0.0001), NKp44 (57 ± 3% vs. 15 ± 3%, P< 0.0001), CD2 (MFI of 25 ± 1.5 vs. 13 ± 1, P=0.004) and LFA-1/CD11a (MFI of 45 ± 1 vs. 29 ± 2, P=0.006) in the IL-15 primed CD56bright NK cells. Due to their known role in activating anti-tumor target responses by NK cells, NKG2D, NKp44, NKp30, CD2, and LFA-1 were evaluated for a non-redundant contribution to the anti-leukemia response of IL-15 primed CD56bright NK cells. Simultaneous blockade of these receptors caused almost complete abrogation of the enhanced anti-leukemic response by the IL-15 primed CD56bright NK cells (Figure 3).
CD56bright NK cells are traditionally considered to poorly respond to leukemia targets. Here we show that stimulation with IL-15 for a few hours markedly enhances their anti-leukemia properties including degranulation and cytotoxicity, as well as IFN-γ and TNF-α production, to a level significantly exceeding CD56dim NK cells. These functional enhancements are explained by multiple mechanisms, including increased cytotoxic effector proteins (perforin, granzyme B, TRAIL), improved leukemia cell conjugation, and enhanced activation requiring LFA-1, CD2 and NKG2D. These results suggest that CD56bright NK cells may play an under-appreciated anti-tumor role in settings of abundant IL-15, such as following lymphodepleting chemotherapy, preparation for stem cell transplantation, or exogenous IL-15 administration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal