Abstract
Neutrophils play a critical role in the innate immune response against various kinds of microbes. In addition to phagocytosis, neutrophil extracellular traps (NETs) have been identified as a novel killing mechanism, capturing microbes with extracellular structures consisting of DNA fibers and antimicrobial granule proteins. On the other hand, aberrant NET formation has been associated with the development of autoimmune and cardiovascular diseases. Various kinds of stimuli, including phorbol myristate acetate (PMA), are known to induce NETs by stimulating the production of reactive oxygen species (ROS), especially singlet oxygen (1O2), through the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) in neutrophils.
Uric acid (UA), a product of purine metabolism, is a scavenger of 1O2 and regulates oxidative stress in humans. UA is believed to suppress Nox-dependent NET formation. By contrast, monosodium urate crystals, an endogenous danger signal, stimulate NET formation in patients with gout, suggesting that a high concentration of UA itself causes NET formation. At present, there are no data explaining the dual action of UA. In this study, we examined the direct effects of UA on NET formation.
Human neutrophils were isolated from the peripheral blood of healthy volunteers and two patients with chronic granulomatous disease (CGD), who provided written informed consent. Detection of 1O2 from the stimulated neutrophils was carried out by chemiluminescence assay using trans-1-(2'-methoxyvinyl)pyrene (MVP) as a 1O2-specific probe. NET formation was analyzed by scanning electron microscope (SEM) or confocal laser microscopy after staining with Sytox Green (dsDNA), and quantified by measuring the Sytox-positive area per cell. Involvement of the NF-κB pathway in NET formation was examined by Western blotting using anti-phospho-NF-κB p65 antibody. Data were compared with Student's t-test, and p values < 0.05 were considered significant.
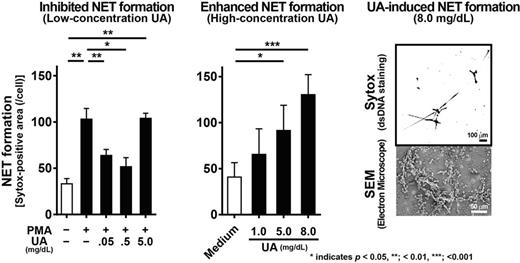
First, we examined the effects of UA on 1O2 production and NET formation by PMA-stimulated neutrophils. The addition of UA (0.05 – 5.0 mg/dL) significantly suppressed 1O2 production by PMA-stimulated neutrophils in a dose-dependent manner. At lower concentrations of UA (≤ 0.5 mg/dL), PMA-induced NET formation was suppressed, as predicted, while a high concentration of UA (5.0 mg/dL) failed to suppress PMA-induced NET formation.
Next, we explored the direct effects of UA on 1O2 production and NET formation. UA treatment alone did not produce any detectable levels of 1O2 or other types of ROS. However, unexpectedly, UA-stimulated neutrophils formed NETs in a dose-dependent manner (1.0 – 8.0 mg/dL of UA). Nox inhibitors (diphenyleneiodonium [DPI] or apocynin) and a 1O2 scavenger (α-phenyl-N-tert-butyl nitrone [PBN]) did not suppress UA-induced NET formation. Moreover, neutrophils in CGD patients produced NETs after treatment with UA, although they had a defect in ROS formation and failed to make NETs after PMA stimulation. These data demonstrate that UA induces NET formation in a Nox-independent manner.
Finally, we explored the signaling pathway in UA-stimulated neutrophils for inducing NET formation. Treatment with NF-κB inhibitor significantly suppressed UA-stimulated NET formation. In addition, using Western blotting, we detected phospho-NF-κB p65 in UA-activated neutrophils. These results indicate that NF-κB activation is indispensable for UA-induced NET formation.
UA suppressed PMA-induced NET formation at low concentrations (≤ 0.5 mg/dL), working as a 1O2 scavenger. By contrast, high concentrations of UA (≥ 5.0 mg/dL) induced NET formation in a Nox- or ROS-independent manner through NF-κB activation. To our knowledge, this is the first study to report on UA-induced NETs. Our findings are clinically significant in that a high level of serum UA may promote the chronic formation of NETs in circulating neutrophils, thereby contributing to vascular damage. Our data may explain one of the missing links between hyperuricemia and the risk of cardiovascular diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal