Abstract
Multiple Myeloma is not a single disease. There is increasing support for risk classification in combination with treatment decision making because of its impact on clinical outcomes. Here we demonstrate additional evidence of the prognostic value of SKY92, an established genetic marker of high risk Multiple Myeloma in a multicenter collection of samples with undisclosed treatments.
A public GEP dataset (MMRC, MMGI portal) contained 114 cases of untreated Multiple Myeloma and was used for SKY92 high risk OS prediction (Kuiper et al. Leukemia 2012). In collaboration with MMRC, OS (with a minimum of at least 2 year follow-up) was collected for 91 of 114 cases for the purpose of this analysis. Briefly, CD138-positive plasma cells had been purified prior to total RNA extraction and subsequent gene expression profiling on Affymetrix U133Plus2.0 GeneChips. The 91 cases represented 9 different clinical sites and their CEL files were normalized as a single batch against a reference cohort of 329 cases after which the SKY92 risk scores were determined as either standard risk or high risk.
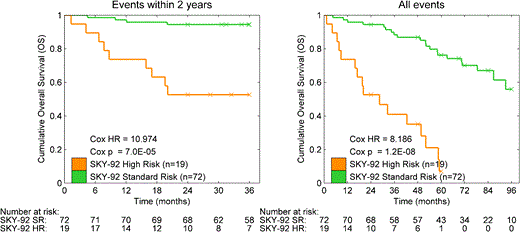
SKY92 resulted in 19 high risk (20.9%) versus 72 standard risk (79.1%) cases in the unselected 91 case-cohort. Comparisons with other high risk GEP signatures will be performed. The OS analysis (Figure 1) shows that the HR cases have significantly shorter survival (Hazard Ratio 11, p = 7 x 10-5). Table 1 shows that high risk patients had more elevated B2M (26.3% vs 13.9%), more low albumin (26.3% vs 16.7%) and more high creatinine (26.3% vs 11.0%). There was no difference between high and standard groups in diagnosis dates (not shown). Cause of the 16 (84.2%) deaths among the high risk cases, and 21 (29.1%) deaths among the standard risk cases indicates that high risk contains less disease progression deaths (57.1% vs 31.3%), and more unknown deaths (56.3% vs 23.8%).
Kaplan-Meier curves for the 91 cases from the MMRC database. Left includes events up to 2 years, right all events.
Kaplan-Meier curves for the 91 cases from the MMRC database. Left includes events up to 2 years, right all events.
The SKY92 classifier identified 19 of 91 cases (21%) as high risk, recapitulating the percentage of high risk in previously studied cohorts (Kuiper et al. 2012). Moreover the hazard ratio of 11 when events up to 24 months or 8.18 when all events are considered, emphasizes the unmet medical need of high risk cases identified with SKY92 as 69% of all deaths within 2 years (9/13 death events) were in this category.
This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine, project BioCHIP grant 03O-102. Rafael Fonseca is a Clinical Investigator of the Damon Runyon Cancer Research Fund. This work is supported by grants R01 CA83724, ECOG CA 21115T, Predolin Foundation, Mayo Clinic Cancer Center and the Mayo Foundation.
van Beers:Skyline Diagnostics: Employment. Van Vliet:Skyline Diagnostics: Employment. Anderson:celgene: Consultancy; onyx: Consultancy; gilead: Consultancy; sanofi aventis: Consultancy; oncopep: Equity Ownership; acetylon: Equity Ownership. Jagannath:Celgene: Honoraria; Millennium: Honoraria. Jakubowiak:BMS: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Millennium: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Onyx: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau. Kumar:Celgene: Clinical Trial Support Other, Membership on an entity’s Board of Directors or advisory committees; Cephalon: Clinical Trial Support, Clinical Trial Support Other; Millennium: Clinical Trial Support, Clinical Trial Support Other, Membership on an entity’s Board of Directors or advisory committees; Novartis: Clinical Trial Support, Clinical Trial Support Other; Onyx: Clinical Trial Support Other, Membership on an entity’s Board of Directors or advisory committees. Lebovic:Celgene: Speakers Bureau; Onyx: Speakers Bureau. Lonial:Millennium: Consultancy; Celgene: Consultancy; Novartis: Consultancy; BMS: Consultancy; Sanofi: Consultancy; Onyx: Consultancy. Reece:Onyx: Honoraria; Novartis: Honoraria; Millennium: Research Funding; Merck: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Research Funding. Siegel:Celgene: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Millennium: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Onyx: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau. Vij:Celgene: Honoraria, Research Funding, Speakers Bureau; Millennium: Honoraria, Speakers Bureau; Onyx: Honoraria, Research Funding, Speakers Bureau. Zimmerman:Celgene: Honoraria; Millennium: Honoraria; Onyx: Honoraria. Fonseca:Medtronic: Consultancy; Otsuka: Consultancy; Celgene: Consultancy; Genzyme: Consultancy; BMS: Consultancy; Lilly: Consultancy; Onyx: Consultancy, Research Funding; Binding Site: Consultancy; Millennium: Consultancy; AMGEN: Consultancy; Cylene: Research Funding; Prognostication of MM based on genetic categorization of the disease: Prognostication of MM based on genetic categorization of the disease, Prognostication of MM based on genetic categorization of the disease Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal