Abstract
Mantle cell lymphoma (MCL) is an aggressive non-Hodgkin's lymphoma (NHL) and represents one of the most challenging lymphoma types to treat. The B-cell antigen Receptor (BCR) signaling pathway is increasingly implicated in the pathogenesis of some B-cell malignancies, and represents an attractive target for therapy. Recent clinical trials have shown that MCL is sensitive to small molecule inhibitors targeting BCR signaling, with response rates reported to be 68% for the BTK-inhibitor Ibrutinib, but only 40% for the PI3Kδ-inhibitor CAL-101. By contrast, chronic lymphocytic leukemia (CLL) has high response rates to both drugs. We hypothesized that basal- or BCR-induced phosphorylation of key molecules that transmit the BCR signal might differ across different NHL, and thereby help us understand therapy responses to small molecular inhibitors.
Single cell flow cytometry measurements of signaling were acquired for samples of MCL (n=34), all obtained prior to therapy. For comparison, samples from CLL (n=14), follicular lymphoma (FL, n=27), diffuse large B-cell lymphoma (DLBCL, n=12), as well as healthy donor peripheral blood (n=8) and tonsils (n=4) were included. BCR-induced signaling was obtained by stimulation with goat anti-human IgM F(ab')2 and IgG F(ab')2 fragments for 4 or 45 minutes. Phosphorylation of 14 signaling molecules was measured under basal (unstimulated) and activation-induced signaling, and CD20, CD3, CD5 and BCL2 were used for discrimination between malignant B cells and tumor infiltrating T cells.
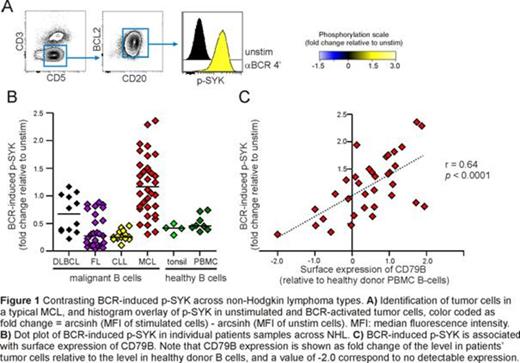
Measurements of basal phosphorylation levels of signaling molecules displayed differences among the NHL types tested. MCL together with DLBCL had highest frequency of patients with tumor cells having elevated levels of p-p65 NF-kB. In contrast, CLL and FL did not have higher basal levels of p-p65 NF-kB. Elevated basal levels of p-Syk and p-AKT1 were most frequently found in CLL and DLBCL, and were less frequent in MCL and not found in FL. In addition, elevated basal level of p-SFK and p-STAT5 were found in a group of MCL patients. BCR-induced signaling in tumor cells showed striking differences across NHL types. For key BCR-signaling molecules including SFK, SYK and PLCg2, BCR-induced phosphorylation was significantly higher in MCL as compared to the other NHL, and was strikingly different from the low levels observed in CLL (Figure 1). Similar results were obtained for Src family kinases (SFK), ERK1/2, AKT1 and STAT5. Although BCR-induced signaling was highly potentiated in most MCL cases, there was a large variation within the MCL cohort. Hence, the fold change of BCR-induced p-PLCg2, relative to unstimulated cells, ranged from 0.28 to 3.01 in MCL tumors, as compared to 0.44 to 0.89 for healthy donor B cells. Furthermore, BCR-induced p-SYK in MCL correlated strongly with p-PLCg2 (r=0.96, p<0.0001), suggesting changes early in the signaling cascade. We therefore measured surface expression of the BCR subunits, IgM and CD79B, and found a strong association between BCR-induced p-SYK and IgM (Figure 1 r=0.65) and BCR-induced p-SYK and CD79B (r=0.64, p<0.0001).
Differences in basal phosphorylation levels of key signaling molecules were found across NHL. BCR-induced signaling was highly potentiated in MCL as compared to the other NHL, and was associated with increased expression of CD79B and IgM. Differences in phosphorylation levels of signaling molecules between different NHL, as well as heterogeneity within each entity might help us understand therapy responses to small molecular inhibitors, although this needs to be tested in follow-up studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal