Background
The outcome for pediatric Non-Hodgkin Lymphomas (NHL) using conventional treatment standards is still inferior compared to other childhood malignancies, particularly in advanced stage and relapsed disease. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an endogenous protein that induces apoptosis selectively in cancer cells by binding to death receptors. Previous TRAIL-targeting strategies have had limited potential due to unfavorable biodistribution and pharmacokinetics. Our laboratory identified a small molecule, ONC201(TIC10), which induces p53 independent TRAIL gene transcription and TRAIL-dependent cell death and overcomes the most critical efficacy-limiting drug properties of available TRAIL-based therapies. Selective advantages of ONC201 include longer half-life, stimulation of TRAIL and death receptor expression, stability, lower production cost, and ability to cross the intact blood-brain barrier. Our lab has reported that ONC201 is orally active, has potent anti-tumor effects in several preclinical animal cancer models including prolonging survival in lymphoma bearing Eu-myc transgenic mice, exhibits synergy with several antitumor agents (Allen JE et al, Sci Transl Med.,2013) and is being developed into a first-in-man study in advanced cancer patients in early 2014.
Assess monoagent activity and mechanism of action of ONC201 in pediatric lymphoma.
Derive preclinical rationale to evaluate ONC201 as a novel targeted therapy for pediatric lymphoma as a monoagent or in combination in clinical trials.
A diverse sub-type panel of human lymphoma cell lines [Ramos, Raji and Daudi (Burkitt's lymphoma); Karpas299 (T-cell NHL); UPN2 and Granta (Mantle Cell lymphoma)] was selected to assess lymphoma sensitivity to ONC201. Luminescent cell viability assay was used to generate inhibition curves at 72 hours post-treatment with ONC201 yielding IC50's. Caspase-based Apoptosis assay was performed on cells treated with low micromolar dose ranges of ONC201 and data generated via flow cytometry at 72 hours post-treatment. Surface TRAIL expression was analyzed via FACS (Fluorescence-activated cell-sorting) at 60 hours post-treatment. Inhibition of ONC201-induced Apoptosis was demonstrated using Pan-caspase inhibitor (Z-VAD-FMK) at 72 hour post-treatment. Western blot analysis of representative cell lines from the three sub-types (Ramos, Karpas299 and UPN2) at 48 hour post-treatment with ONC201 for AKT and ERK was used to validate the mechanism of induction of apoptosis of lymphoma cells by ONC201.
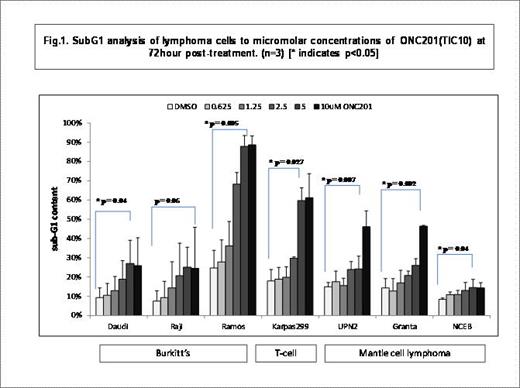
Cell viability assays demonstrated sharp dose-response curves at 72 hours post-treatment with ONC201. Quantitative analysis of these relationships yielded ONC201 IC50 values in the range of 1.3 to 5.06 uM, which is comparable to reported activity of ONC201 in other tumor types. Sub-G1 analysis by flow cytometry revealed significant levels of apoptosis at 72 hours post-treatment in a dose-dependent manner (Fig.1) with substantial increase in sub-G1 content (range 1.8-fold to 4.15-fold) in comparison with that of untreated cells. Treatment with ONC201 revealed increase in surface TRAIL expression in a dose dependent manner across the representative cell lines. ONC201 induced apoptosis was demonstrably inhibited using Pan-caspase inhibitor. Western blot analysis of representative cell lines from the three sub-types (Ramos, Karpas299 and UPN2) at 48 hour post-treatment with ONC201 confirmed dual inhibition of Akt and ERK as potential mechanism of induction of apoptosis of lymphoma cells by ONC201.
ONC201 is a first-in-class therapy that has apoptotic activity in human lymphoma cells at low micromolar concentrations and promotes up-regulation of TRAIL. ONC201 is promising as a monoagent and may be enhanced by combination with approved therapies to improve the standard of care for pediatric NHL. We are currently assessing in vitro synergistic tumor cell-killing by standard chemotherapeutic agents used to treat lymphoma in combination with ONC201. We plan to translate our work in vivo using xenograft mice models. The ultimate goal of this project is to provide the preclinical rationale for a potential phase Ib trial of ONC201 as a combination therapy in pediatric lymphoma.
Allen:ONCOCEUTICS: Employment; ONCOCEUTICS: Equity Ownership. El-Deiry:ONCOCEUTICS: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal