Abstract
Despite increasing insights into its immunobiology, graft vs host disease (GVHD) remains a major obstacle for successful allogeneic hematopoietic stem/progenitor cell transplantation (allo-HCT). Separation of GVHD from graft vs. leukemia/lymphoma (GVL) responses also remains an elusive goal for allo-HSCT. Efforts to delineate the transcriptional networks regulating T cell differentiation post-HCT have suggested that multiple transcription factors may be involved in the regulation of alloreactive helper T (Th) cells and GVHD. However, conflicting data have emerged regarding the role of Th1 and Th17 pathways, and it remains unclear which transcription factors mediate the early activation of alloreactive T cells necessary for subsequent GVHD development. The T-box transcription factor eomesodermin (Eomes) cooperates with T-bet to regulate CD8 T cell cytotoxic function, IFNy production, and memory cell formation. Recently, a role for Eomes in CD4 Th cell polarization has been described as well.
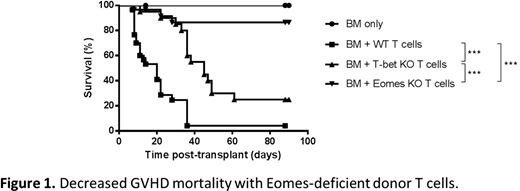
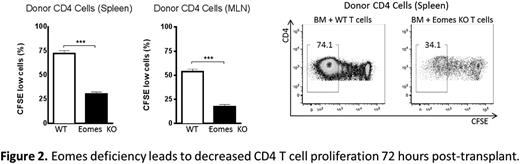
In order to evaluate the role of Eomes in T cell function in the context of allo-HCT, we used a MHC-disparate mouse model (C57BL/6 into BALB/c) with T cell depleted donor bone marrow (TCD-BM) and wild-type (WT) or Eomes knock out (KO) donor T cells. Recipients were conditioned with lethal total body irradiation. Eomes deficiency in donor T cells led to a significant reduction in GVHD mortality (Fig 1, p<.001), morbidity (p<.001), and intestinal pathology (p<.05, colon). Notably, Eomes KO T cells exerted significantly less GVHD mortality than T-bet KO T cells (Fig 1, p<.001). Given the reduced gastrointestinal (GI) GVHD observed with Eomes KO T cells, we next analyzed the expression of homing molecules important for T cell migration to the GI tract. Consistent with reduced GI GVHD, we detected reduced expression of α4β7 integrin on Eomes KO donor CD8 T cells one week post-HCT. We also observed an increase in the proportion and absolute numbers of Foxp3+ regulatory T cells, as well as a decrease in expression of T-bet in mesenteric lymph nodes (MLNs). Moreover, we found decreased production of IFNy by Eomes KO donor CD4 T cells two weeks (spleen and MLN, p<.001) and three weeks (spleen, p<.01) post-HCT without a comcomitant increase in IL-17. We also found increased IL-4 production by Eomes KO CD4 T cells two weeks post-HCT (MLN, p<.05), indicating a shift from Th1 to Th2 polarization in the absence of Eomes. Strikingly, one of the greatest differences we observed between WT and Eomes KO donor T cells was impaired early activation of CD4 T cells; Eomes deficiency was associated with reduced proliferation (p<.001), reduced expression of CD25 (p<.001, spleen; p<.001, MLN), and increased expression of CD62L (p<.01, spleen; p<.001, MLN) in CD4 T cells within the first 72 hours post-HCT (Fig 2).
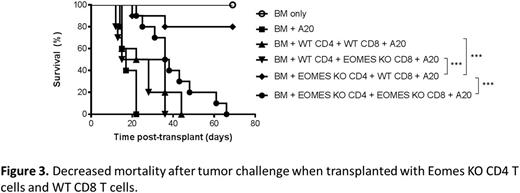
In order to determine if Eomes was important for T cell-mediated GVL responses, we performed allo-HCT in the presence of A20 lymphoma cells. Despite the reduction in GVHD mortality as described above, A20 tumor challenge led to increased mortality in recipients of Eomes KO T cells, indicating that Eomes was also critical for effective GVL function. Given the importance of Eomes in early alloactivation of CD4 T cells, we evaluated if the impaired GVL function was due to an intrinsic CD8 defect or lack of CD4 help. B6 TCD-BM was transplanted into BALB/c recipients along with either WT or Eomes KO CD4 or CD8 T cells. Eomes deficiency in both CD4 and CD8 T cells again led to significant mortality, but HCT with Eomes KO CD4 T cells and WT CD8 T cells led to the greatest survival due to less GVHD and intact GVL (Fig 3), suggesting that Eomes is essential for intrinsic CD8 function during GVL, but not for CD4 help.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal