Abstract
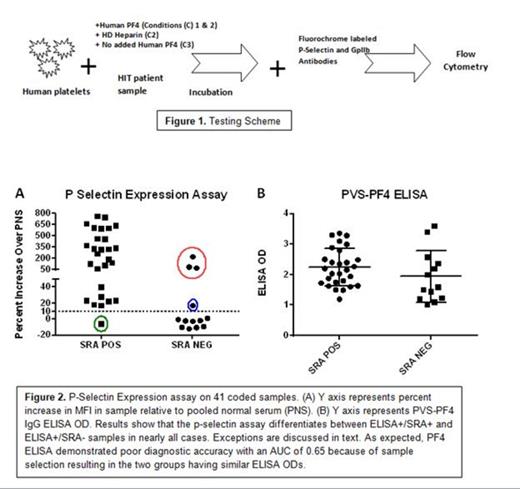
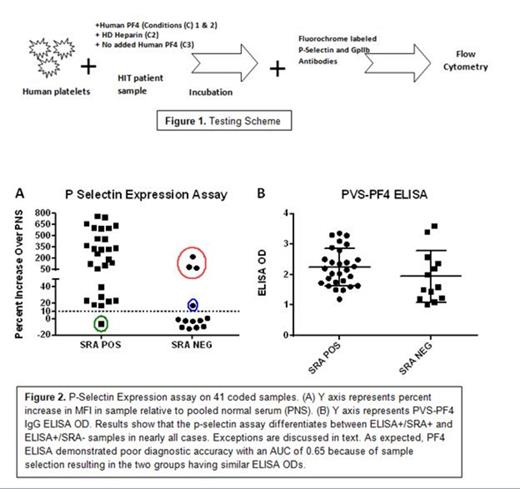
Heparin induced thrombocytopenia and thrombosis (HIT) is a major cause of morbidity and mortality in patients given heparin and accurate and timely diagnosis is critical for successful management of this condition. HIT is caused by antibodies specific for a basic platelet protein, PF4, in a complex with heparin. An assay widely used to detect such antibodies, the PF4-based ELISA, is highly sensitive for antibody detection but not specific for the clinical disorder, HIT. An alternative test, the serotonin release assay (SRA) is quite specific for the clinical disorder, but is demanding technically and is performed routinely only by a few laboratories. Assigning a HIT diagnosis wrongly (due to lack of diagnostic specificity in the ELISA) can lead to treatment with alternative anticoagulants which are expensive, carry higher bleeding risks, and (unlike heparin) lack a specific antidote. Here, we describe a newly developed assay that has operating characteristics similar to the SRA in that it preferentially recognizes “activating” antibodies likely to be pathogenic but is much less demanding technically. The assay end point for platelet activation is FcγRIIa-mediated expression of p-selectin (CD62p) triggered by binding of antibody to PF4 in a complex with platelet surface glycosaminoglycans, a process that mimics the mechanism by which HIT antibodies are thought to activate platelets and predispose to thrombosis in vivo. The unique feature of the assay is addition of exogenous PF4 to platelets, an approach not previously used in diagnostic testing for HIT (Fig. 1). We compared test performance using a set of blinded HIT samples that were either SRA positive or SRA negative, but reacted equally well in the PF4 ELISA. The new assay correctly identified “pathogenic” HIT antibodies (i.e. those positive in the SRA) in 36 of 41 patient samples using a threshold of >10% increase in p-selectin expression relative to pooled negative serum (Fig 2). Five samples were originally “misclassified”, with the p-selectin expression assay having an area under the curve (AUC, a measure of diagnostic accuracy) of 0.90. Four samples were “false positives” i.e., were SRA negative but positive in the p-selectin assay (Fig 2, Red and blue circles) and one was “false negative” (Fig 2, Green circle). Three of the four “false positives” (red circle) reacted strongly in the p-selectin assay, were inhibited with high dose heparin and failed to react in the absence of added PF4 (data not shown). These findings strongly suggested the presence of a heparin-depending antibody in these 3 samples, and repeat SRA demonstrated that all three were actually SRA+. Thus, 39 of 41 HIT samples produced test results concordant with SRA, with an AUC for the new assay of 0.98 (1.0 indicates “perfect” performance). We postulate that the ability of the p-selectin expression assay to preferentially identify HIT antibodies that activate platelets is a consequence of the close similarity between the mechanics of the test and the pathogenic process that takes place in vivo in a patient with HIT. Testing of a larger prospective series of HIT samples to further assess diagnostic utility of the assay is in progress.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal