Key Points
C3aR activation increases ATP efflux, NLRP3 inflammasome activation, and IL-1β secretion in human monocytes.
C3aR-activated monocytes drive Th17 responses in vitro and likely in vivo.
Abstract
Interleukin-1β (IL-1β) is a proinflammatory cytokine and a therapeutic target in several chronic autoimmune states. Monocytes and macrophages are the major sources of IL-1β. IL-1β production by these cells requires Toll-like receptor (TLR) and adenosine triphosphate (ATP)–mediated P2X purinoceptor 7 (P2X7) signals, which together activate the inflammasome. However, how TLR signals and ATP availability are regulated during monocyte activation is unclear and the involvement of another danger signal system has been proposed. Here, we demonstrate that both lipopolysaccharide (LPS) and the anaphylatoxin C3a are needed for IL-1β production in human macrophages and dendritic cells, while in monocytes, C3a enhanced the secretion of LPS-induced IL-1β. C3a and LPS-stimulated monocytes increased T helper 17 (Th17) cell induction in vitro, and human rejecting, but not nonrejecting, kidney transplant biopsies were characterized by local generation of C3a and monocyte and Th17 cell infiltration. Mechanistically, C3a drives IL-1β production in monocytes by controlling the release of intracellular ATP into the extracellular space via regulation of as-yet unidentified ATP-releasing channels in an extracellular signal-regulated kinase 1/2–dependent fashion. These data define a novel function for complement in inflammasome activation in monocytes and suggest that C3aR-mediated signaling is a vital component of the IL-1β–Th17 axis.
Introduction
Interleukin-1β (IL-1β) is a key proinflammatory cytokine involved in host responses to pathogens and tissue injury. IL-1β has gathered substantial interest in recent years because several autoimmune disorders respond specifically to IL-1 receptor blockade using either soluble IL-1 receptor antagonists or blocking monoclonal antibodies (mAbs).1 In addition to inducing neutrophil activation and histamine release by mast cells and maintaining epithelial cell integrity,2 IL-1β also plays a central role in modulating adaptive effector T-cell responses by preferentially inducing T helper 1 (Th1) and Th17 differentiation.3 In humans, CD4+ T cells require IL-1β and IL-6 to differentiate into Th17 cells,4 which contribute to the pathophysiology of inflammatory and autoimmune diseases5 as well as ischemia reperfusion injury (IRI) and transplant rejection.6
Monocytes, macrophages, and dendritic cells (DCs) are major IL-1β sources and release this cytokine in response to stimuli such as pathogen-associated or danger-associated molecular patterns (PAMPs or DAMPs) mediated by signaling via several Toll-like receptor (TLR) pathways.7 Although consensus exists that IL-1β generation requires processing of the 31 KD inactive pro–IL-1β to the 17 KD active form by caspase-1 activation via an inflammasome complex (eg, NLRP3),8,9 signals leading to NLRP3 and caspase-1 activation are not fully understood. Beside TLR activation, additional stimuli contribute to NLRP3 assembly, including elevated extracellular glucose, hyaluronan, uric acid, and monosodium urate crystals.8 Importantly, in both monocytes10 and macrophages11 activation of the adenosine triphosphate (ATP)–gated ion channel purinoceptor 7 (P2X7) by ATP has been shown to significantly enhance the production of TLR4 (lipopolysaccharide [LPS])–induced IL-1β. In fact, in macrophages, concomitant stimulation with ATP and LPS is essential for IL-1β production.12 ATP efflux into the extracellular space and subsequent P2X7 activation can be regulated by several channel systems including connexin and pannexin hemichannels, anion channels, as well as by lysosome exocytosis.13-17
The complement system is part of the innate arm of immunity and considered an archetypical danger sensing system.18 Pathogen recognition by complement leads to the activation of this system in a cascade-like fashion and the generation of its most potent effector molecules, the opsonin C3b and the anaphylatoxins C3a and C5a. Similar to TLRs, complement is activated not only by PAMPs but also by DAMPs.18 Not surprisingly, crosstalk between complement and TLRs has been described in several cell types.19,20 Particularly, signals mediated through the C3a receptor (C3aR) and C5a receptor (C5aR) expressed on macrophages and DCs are known to be either regulated by incoming TLR signals or, conversely, regulate TLR-mediated signals and impact on the cytokine release by these cells.19-21 We therefore hypothesized that complement activation fragments such as C3a and C5a participate in the induction of IL-1β production and aimed to assess the potential molecular mechanisms. A role for C3a in IL-1β secretion by monocytes had been indicated previously22 ; however, the results observed were acknowledged to be potentially confounded by trace LPS contamination of the C3a preparations.23 Furthermore, the C3aR-mediated signaling events linking complement and IL-1β production are entirely unexplored. We demonstrate here that monocytes (when compared with macrophages and DCs) proved to be the highest source of IL-1β and while LPS alone was able to induce IL-1β in monocytes, additional C3a significantly increased their production of this cytokine. Modulation of IL-1β expression in monocytes by C3aR signaling is mediated by extracellular signal-regulated kinase 1/2 (ERK1/2)–dependent enhanced ATP efflux into the extracellular space and subsequent engagement of P2X7 with downstream NLRP3 and caspase-1 activation. Furthermore, such C3aR-induced increase in IL-1β secretion by monocytes fosters differentiation of Th17 effector T cells in in vitro cultures.
Methods
Healthy donor and kidney biopsy samples
Cells were isolated from buffy coats (National Blood Service) or blood samples from healthy volunteers. Informed consent was obtained from all subjects and blood was collected and processed with the approval of the King’s College Ethics Committee guidelines (reference no. 09/H0804/72). Renal biopsy samples were from patients who had given consent to use of biopsy material surplus to diagnostic requirements. Informed consent was obtained in accordance with the Declaration of Helsinki.
Abs, proteins, and activating or inhibitory reagents
Anti-CD3 (OKT-3) was prepared in-house from a hybridoma line and the anti-CD28 (CD28.1) Ab was purchased from BD Biosciences, as were Abs to CD80 (L307.4), CD86 (IT2.2), and CD120 (MABTNFR1-B1). Abs to IL-1β (3A6), ERK1/2 (137F5), and phosphorylated ERK1/2 (D13.14.4E) were from Cell Signaling Technologies. Anti–Caspase-1 (ab17820), anti–β-actin (ab8226), and anti-TLR4 (ab22048) were from Abcam and anti-NLRP3 (AG-20B-0014) was from AdipoGen. Anti–IL-4 (MP4-25D2) and anti–interferon-γ (anti–IFN-γ) (NIB-42) Abs were obtained from eBioscience. Anti-C5aR Ab (sc-53795) was from Santa Cruz Biotechnology and anti-C3aR Ab (345804) was from Biolegend. Abs to CD4 (BC/1F6), IL-17 (polyclonal), and C3a (4H3) were purchased from Abcam. Polyclonal antisera to pannexin-1 (ab60098 and ab139715; Abcam) were bought from Invitrogen.
Recombinant human IL-2 was a gift from Dr Christine Pham (Washington University, St. Louis, MO); recombinant (r) IL-4, IL-6, IL-1β, and IL-23 were purchased from R&D Systems. Macrophage and granulocyte macrophage colony-stimulating factors (M-CSF and GM-CSF) were from Invitrogen. Recombinant human C5a (234397) was from Calbiochem, and serum-purified human C3a (A118) was from CompTech. Recombinant soluble human IL-1 receptor-like 1 (C-61122) was purchased from Promokine. The C3aR nonpeptide agonist (C4494), LPS, and ATP (A1852), oxidized ATP (oxATP, A6779), Carbenoxolone (CBX) (C4790), and nigericin (N7143) were from Sigma-Aldrich; the Caspase-1 inhibitor (400015) was bought from Calbiochem. The P2X7 receptor antagonist AZ 11645373 and Probenecid (4107) were bought from Tocris Biosciences, apyrase (M0393L) from New England BioLabs, Inc., and the MEK inhibitor from Calbiochem.
Monocyte and T-cell purification, macrophage and DC generation, and cell activation
CD4+ T cells and monocytes were purified from blood using magnetic CD4+ or CD14+ microbeads, respectively (Miltenyi Biotec). Cell purity and viability was consistently >95%. To generate macrophages or DCs, purified monocytes were cultured in 24-well plates in RPMI 1640 media containing 50 ng/mL rM-CSF or rIL-4 and rGM-CSF, respectively. Media was replaced every 2 to 3 days and matured macrophages or DCs were used at days 5 to 6 for experiments. Macrophage/DC generation was confirmed by monitoring CD14 downregulation and major histocompatibility complex II, CD80, CD86, and CD120 (macrophages only) upregulation. Monocytes, macrophages and DCs were activated in 24-well plates with LPS (100 ng/mL). T cells were activated in 48-well plates coated with Abs to CD3 and CD28 (2 μg/mL phosphate-buffered saline [PBS]) and addition of rIL-2. For Th17-skewing conditions, rIL-6 (25 ng/mL), rIL-23 (25 ng/mL), and rIL-1β (10 ng/mL), and function-neutralizing Abs to IL-4 (10 µg/mL) and IFN-γ (10 µg/mL), were added.
ATP, membrane permeability, viability, and caspase-1 measurements
ATP was measured using either the ENLITEN ATP Assay System Bioluminescence Detection kit (Promega) or the ATP Assay kit (Abcam). Cellular flux and viability was assessed with the Membrane Permeability/Dead Cell Apoptosis kit with YO-PRO-1 and the PI kit from Invitrogen. Caspase-1 activation was measured using the Green FLICA Caspase-1 Assay kit (Immunochemistry Technologies).
Cytokine measurements
IL-1β, IL-6, IL-18, and IL-23 were measured using the appropriate enzyme-linked immunosorbent assay (ELISA) kits from eBioscience. Th1, Th2, and Th17 cytokine secretion was assessed using the BD Biosciences Cytokine Bead Array. Intracellular IL-17 was determined after cell restimulation with 50 ng/mL phorbol myristate acetate and 500 ng/mL ionomycin (Sigma-Aldrich) for 4 hours in the presence of monensin (Biolegend).
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples were deparaffinized and hydrated through decreasing concentrations of ethanol. Heat-induced antigen retrieval was conducted in 10 mM sodium citrate solution (pH 6.0) for 20 minutes in a microwave. Slides were washed 3 times in PBS, and incubated with 5% bovine serum albumin for 60 minutes. Primary Abs were diluted to the manufacturers’ recommended concentrations and applied for overnight incubation at 4°C. Secondary Ab staining was conducted for 60 minutes at room temperature either using fluorochrome-labeled Abs for fluorescence microscopy or horseradish peroxidase–labeled Abs in conjunction with the Vectastatin Elite ABC kit (Vector Laboratories Inc) for light microscopy. Nuclei were counterstained with 4,6 diamidino-2-phenylindole (DAPI) for fluorescence microscopy or with hematoxylin. Confocal images were taken at 22°C using the A1R Si confocal microscope and NIS-Elements C software (both from Nikon).
Statistical analysis
Statistical analyses were performed using analysis of variance (ANOVA) or the paired t test and Bonferroni correction where appropriate (Graphpad Prism, GraphPad Software, Inc.; and Excel software, Microsoft).
Results
C3aR engagement mediates IL-1β production by monocytes, macrophages, and DCs
To assess the contributions of C3aR and TLR4 stimulation on IL-1β secretion by human monocytes, macrophages, or DCs, cells were cultured in media or in media supplemented with a C3aR agonist, LPS, or a combination thereof. A chemical C3aR agonist was used to limit the possibility of LPS contamination during the C3a serum purification process23 and to ensure specificity as C3a has been shown to also activate the C5aR when present in high concentrations.24 In monocytes, while addition of LPS induced IL-1β as previously reported,12 C3aR activation alone did not lead to IL-1β generation (Figure 1A). However, the combination of TLR4 and C3aR-mediated stimulation increased monocytic IL-1β production by approximately fourfold. The effect of the C3aR agonist on LPS-mediated IL-1β production is specific as C3aR activation did not modulate the secretion of 2 other typical monocyte cytokines, IL-6 and IL-23; C5a stimulation did not impact on IL-1β generation but induced IL-6 secretion by monocytes as previously described (Figure 1A).25 Importantly, serum-purified C3a at a physiological range26 had a comparable effect on IL-1β (supplemental Figure 1, available on the Blood website), IL-6, and IL-23 production (data not shown), suggesting that C3aR agonist and C3a mediate similar signals.
C3a significantly increases LPS-mediated IL-1β production by human monocytes. (A) IL-1β, IL4-6, and IL-23 production of monocytes measured by ELISA after LPS and anaphylatoxin activation. Monocytes were incubated for 24 hours in media or in media with addition of LPS (100 ng/mL) and with or without addition of human C3aR agonist (C3aRag, 25 μM) or purified human C5a (50nM). Data represent mean ± SD from 3 independently performed experiments (n = 3). (B-C) Kinetic of IL-1β secretion by activated monocytes without (B) or with 2 hours of 100 ng/mL LPS priming (C) before LPS and C3aRag addition. Data represent the mean values derived from 2 separate experiments with conditions performed in triplicate. (D) Western blot analyses for IL-1β precursor and mature protein forms of cell lysates at 4 hours postactivation (left panel) and of concentrated cell supernatants from monocytes that had been activated for 8 hours (right panel). (E-F) Western blot analyses of cell lysates from 8-hour activated monocytes assessed for NLRP3 protein content (E) and presence of the 20-kDa active caspase-1 fragment (F). β-actin levels were measured as loading controls. Data in panels D-F are representative of 3 independently performed experiments using a different healthy donor each time. *P < .05; **P < .005; ***P < .001 as determined by the paired t test with Bonferroni correction.
C3a significantly increases LPS-mediated IL-1β production by human monocytes. (A) IL-1β, IL4-6, and IL-23 production of monocytes measured by ELISA after LPS and anaphylatoxin activation. Monocytes were incubated for 24 hours in media or in media with addition of LPS (100 ng/mL) and with or without addition of human C3aR agonist (C3aRag, 25 μM) or purified human C5a (50nM). Data represent mean ± SD from 3 independently performed experiments (n = 3). (B-C) Kinetic of IL-1β secretion by activated monocytes without (B) or with 2 hours of 100 ng/mL LPS priming (C) before LPS and C3aRag addition. Data represent the mean values derived from 2 separate experiments with conditions performed in triplicate. (D) Western blot analyses for IL-1β precursor and mature protein forms of cell lysates at 4 hours postactivation (left panel) and of concentrated cell supernatants from monocytes that had been activated for 8 hours (right panel). (E-F) Western blot analyses of cell lysates from 8-hour activated monocytes assessed for NLRP3 protein content (E) and presence of the 20-kDa active caspase-1 fragment (F). β-actin levels were measured as loading controls. Data in panels D-F are representative of 3 independently performed experiments using a different healthy donor each time. *P < .05; **P < .005; ***P < .001 as determined by the paired t test with Bonferroni correction.
Interestingly, the same activation conditions applied to macrophages and DCs demonstrated that IL-1β, IL-6, and IL-23 production by these cells require distinct signals: IL-1β (and IL-23) production by macrophages and DCs needed simultaneous TLR4 and C3aR engagement and was absent using LPS alone at any time point measured (supplemental Figure 2A-B). In addition, the concentrations of cytokines secreted by these cells were significantly less compared with those from monocytes, which produced 10-fold more IL-1β and IL-23 and twice the amount of IL-6. All cell populations expressed TLR4 and the anaphylatoxin receptors; however, expression of TLR4 was downregulated on DCs and C3aR expression was substantially lower on macrophages and DCs compared with monocytes (supplemental Figure 3A-C). Reduced/low C3aR presence is fully in line with previous findings on tissue-resident human DCs27 and could potentially explain the differences in cytokine production. Because monocytes proved to be the main source of IL-1β, subsequent experiments focused on these cells. LPS or LPS+C3aR agonist-driven IL-1β production by monocytes peaked 8 hours postactivation and remained stable for at least 24 hours (Figure 1B). Priming of monocytes with LPS prior to C3aR agonist addition induced earlier IL-1β production but did not affect the total amounts secreted by activated cells (Figure 1C). Western blot analyses of cell supernatants confirmed the presence of the processed active 17 KD form of IL-1β at 8 hours postactivation in LPS and in LPS+C3aR agonist-treated cultures (Figure 1D). In line with this, increased expression of NLRP3 protein (Figure 1E) and generation of activated caspase-1 (Figure 1F; supplemental Figure 4A), both critical mediators of IL-1β generation,8,9 were observed in the lysates of these cells. Conversely, the addition of a caspase-1 inhibitor reduced LPS-induced IL-1β production, and had a clear inhibitory effect on LPS+C3aR-mediated secretion of IL-1β (supplemental Figure 4B).
These data suggest that C3aR coengagement increases LPS-induced IL-1β production in monocytes by potentiating NLRP3 assembly and caspase-1 activation.
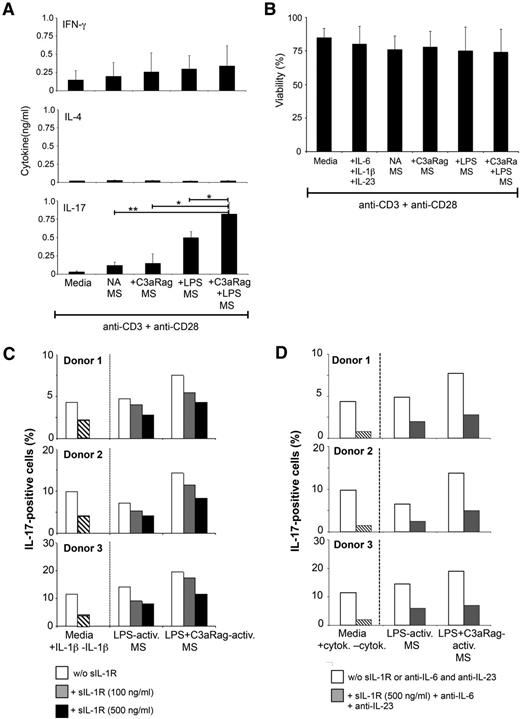
C3a-activated monocytes induce Th17 responses
Considering that IL-1β, together with IL-6 and IL-23 drive Th17 responses,4 we next assessed the effect of C3aR-activated monocytes on CD4+ T-cell–derived effector cytokine production. Activation of T cells in the presence of conditioned media from monocytes activated by C3aR agonist, LPS, or LPS+C3aR agonist, demonstrated that the latter 2 activation conditions induced a significant increase in IL-17 secretion, while Th2 cytokine production was unaffected, and IFN-γ (Th1) secretion was only marginally increased (Figure 2A). None of the activation conditions impacted on T-cell viability (Figure 2B). Furthermore, increased IL-17 presence induced by LPS and LPS+C3aR agonist correlated with the absolute numbers of IL-17+ cells generated in cultures (supplemental Figure 5). Functional neutralization of IL-1β in the conditioned media by addition of soluble recombinant IL-1 receptor decreased Th17 induction in a dose-dependent manner by 30% to 45% in all conditions tested (Figure 2C). These data indicate that the C3a-mediated increase in IL-1β production by monocytes translates into amplified Th17 responses. However, an additional unidentified factor may contribute to induction of IL-17 responses, as simultaneous neutralization of IL-1β, IL-6, and IL-23 only reduced Th17 induction by ∼60% (Figure 2D).
C3aR-activated monocytes induce increased Th17 responses. (A) Human CD4+ T cells respond to supernatants from LPS+C3aR-activated monocytes with increased IL-17 secretion. CD4+ T cells were activated with immobilized Abs to CD3 and CD28 in the presence of fresh media or supernatants derived from monocytes that had not been activated or activated with C3a agonist (C3aRag) alone, LPS, or LPS and C3aRag. Induction of Th1 (IFN-γ), Th2 (IL-4), and Th17 (IL-17) responses was assessed 3 days postactivation using the Cytokine Bead Array. (B) T-cell viability is unaffected by exposure to monocyte-derived supernatants. Experiments were performed as described in panel A but with inclusion of the Th17-skewing control condition (+IL-6, IL-1β, and IL-23) and cell viability was assessed 3 days post–T-cell activation. Data shown in panels A-B represent mean ± SD from 4 independently performed experiments (n = 4). (C) Functional inhibition of C3a-induced monocyte-derived IL-1β significantly decreases Th17 induction. Experiments were performed as in panel A but in the absence or presence of increasing amounts of soluble IL-1 receptor (sIL-1R), which functions as an IL-1R antagonist. Control cells (2 left bars) were activated in the presence of rIL-6 and rIL-23 but with or without rIL-1β. Bars represent the median values of each condition performed in duplicate with monocyte-derived supernatants from 3 distinct donors and with T cells isolated from 3 additional different donors (n = 3). (D) Neutralization of IL-1β, IL-6, and IL-23 does not abrogate Th17 induction. Experiments were performed as in panel A but in the absence or presence of 500 ng/mL sIL-1R and function-neutralizing mAbs to IL-6 and IL-23 (10 μg/mL each). Control cells (2 left bars) were activated either in the presence or absence of Th17-skewing cytokines. *P < .05; **P < .005 as determined by ANOVA. activ, activated; cytok, cytokine; MS, monocyte-derived supernatants; NA, nonactivated.
C3aR-activated monocytes induce increased Th17 responses. (A) Human CD4+ T cells respond to supernatants from LPS+C3aR-activated monocytes with increased IL-17 secretion. CD4+ T cells were activated with immobilized Abs to CD3 and CD28 in the presence of fresh media or supernatants derived from monocytes that had not been activated or activated with C3a agonist (C3aRag) alone, LPS, or LPS and C3aRag. Induction of Th1 (IFN-γ), Th2 (IL-4), and Th17 (IL-17) responses was assessed 3 days postactivation using the Cytokine Bead Array. (B) T-cell viability is unaffected by exposure to monocyte-derived supernatants. Experiments were performed as described in panel A but with inclusion of the Th17-skewing control condition (+IL-6, IL-1β, and IL-23) and cell viability was assessed 3 days post–T-cell activation. Data shown in panels A-B represent mean ± SD from 4 independently performed experiments (n = 4). (C) Functional inhibition of C3a-induced monocyte-derived IL-1β significantly decreases Th17 induction. Experiments were performed as in panel A but in the absence or presence of increasing amounts of soluble IL-1 receptor (sIL-1R), which functions as an IL-1R antagonist. Control cells (2 left bars) were activated in the presence of rIL-6 and rIL-23 but with or without rIL-1β. Bars represent the median values of each condition performed in duplicate with monocyte-derived supernatants from 3 distinct donors and with T cells isolated from 3 additional different donors (n = 3). (D) Neutralization of IL-1β, IL-6, and IL-23 does not abrogate Th17 induction. Experiments were performed as in panel A but in the absence or presence of 500 ng/mL sIL-1R and function-neutralizing mAbs to IL-6 and IL-23 (10 μg/mL each). Control cells (2 left bars) were activated either in the presence or absence of Th17-skewing cytokines. *P < .05; **P < .005 as determined by ANOVA. activ, activated; cytok, cytokine; MS, monocyte-derived supernatants; NA, nonactivated.
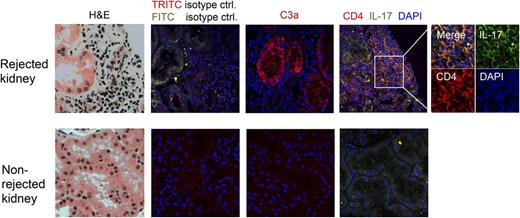
Deposition of complement activation fragments onto graft epithelial and endothelial cells is a strong contributor to IRI and transplant rejection,28 as are infiltration of cytokine-producing monocytes29 and increased levels of IL-17 (beside IFN-γ).30,31 In renal transplants, tubular epithelial cells and infiltrating CD4+ T cells are known sources of IL-17.30,31 Interestingly, although the generation of C3a is assumed in graft rejection, it has hitherto not been assessed in inflamed human tissue. We therefore evaluated whether biopsy samples from patients with T-cell–mediated acutely rejecting renal transplants contain the “players” required for a complement-driven local Th17 induction axis, that is, C3a, monocytes, and IL-17+CD4+ T cells. C3a generation was determined using an Ab that only recognizes the cleaved C3a neoepitope and not C3a contained within the C3 α-chain. Control nonrejecting tissue showed neither lymphocyte infiltration nor C3a presence (Figure 3 bottom panels). In contrast, tissue samples from acutely rejecting kidneys were characterized by high numbers of infiltrating CD4+ lymphocytes of which about 20% to 25% were IL-17+ (Figure 3 top panel; supplemental Figure 6A) and strong C3a staining colocalizing with tubular epithelial cells in the regions of leukocyte infiltration (Figure 3 top panel). CD68+ cells consistent with monocytes or macrophages were observed only in the rejecting grafts and also colocalized with the T-cell infiltrates (supplemental Figure 6B). These data support a scenario in which monocytes migrating into sites of inflammation encounter a C3a-rich environment and subsequently induce a Th17 phenotype in infiltrating lymphocytes.
Biopsies from human kidney transplants with acute T-cell–mediated rejection are characterized by high tubular C3a production and IL-17 infiltration. Histology samples from acutely rejecting (top panels) and nonrejecting (bottom panels) kidney transplants were stained with indicated Abs and analyzed by confocal fluorescence microscopy. Results shown are representative of histology data obtained from samples of 3 patients with acute kidney rejection and 2 patients without signs of kidney rejection (×60 magnification for the 8 large panels, and ×100 magnification for the inset). ctrl, control; FITC, fluorescein isothiocyanate; H&E, hematoxylin and eosin; TRITC, tetramethylrhodamine isothiocyanate.
Biopsies from human kidney transplants with acute T-cell–mediated rejection are characterized by high tubular C3a production and IL-17 infiltration. Histology samples from acutely rejecting (top panels) and nonrejecting (bottom panels) kidney transplants were stained with indicated Abs and analyzed by confocal fluorescence microscopy. Results shown are representative of histology data obtained from samples of 3 patients with acute kidney rejection and 2 patients without signs of kidney rejection (×60 magnification for the 8 large panels, and ×100 magnification for the inset). ctrl, control; FITC, fluorescein isothiocyanate; H&E, hematoxylin and eosin; TRITC, tetramethylrhodamine isothiocyanate.
C3a regulates ATP efflux in monocytes
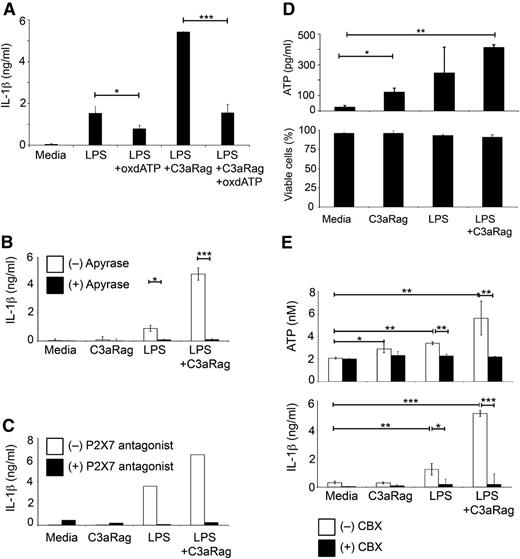
TLR and ATP-mediated signals (via P2X7 activation) induce the assembly of the NLRP3 inflammasome, which contains activated caspase-1 required for the generation of active IL-1β.8,11 Intracellular signaling events downstream of C3aR activation in human monocytes, however, are unexplored. Because C3aR engagement on DCs decreases cAMP levels,32 and cAMP is generated from ATP via adenylate cyclase activity, a potential connection between C3a and ATP generation was explored. Indeed, treatment of monocytes with oxATP abrogated the C3a-mediated increase in IL-1β production and reduced LPS-induced IL-1β release by ∼50% (Figure 4A). Additionally, media supplementation with either apyrase, which catalyses the hydrolysis of ATP to AMP, or a selective P2X7 antagonist abolished IL-1β secretion in all conditions (Figure 4B-C). Correspondingly, coengagement of TLR4 and C3aR induced the highest amount of extracellular ATP in monocyte cultures, while C3aR activation and LPS activation alone induced, low and intermediate ATP efflux, respectively (Figure 4D top panel). This extracellular increase in ATP is not due to “leakage” from apoptotic cells, as monocyte cell viability is comparable among activation conditions (Figure 4D bottom panel). Efflux of intracellular ATP into the extracellular environment is regulated by several mechanisms including the assembly of connexin 43 and pannexin-1 hemichannels.15-17 CBX can block some of these channel systems, and CBX supplementation of media indeed led to a significant decrease in extracellular ATP and to abolition of IL-1β secretion in LPS and in LPS+C3aR agonist-treated cultures (Figure 4E). These results suggest that C3aR activation in monocytes increases cellular ATP efflux via a CBX-sensitive channel system.
C3aR-mediated increase in IL-1β production is regulated via ATP cell efflux. (A-C) Monocyte treatment with oxATP, with apyrase or with a specific P2X7 antagonist abrogates LPS and LPS+C3a agonist-mediated IL-1β production. Monocytes were either left untreated or treated for 30 minutes with 300 μM oxATP (A), with 2.5 units/mL apyrase (B), or with 1 μM of the specific P2X7 antagonist AZ11645373 (C) before 24-hour activation with LPS and C3aR agonist (C3aRag) and subsequent assessment of IL-1β secretion. (D) C3aR activation increases extracellular ATP but does not affect cell viability. Freshly purified monocytes were activated as depicted (LPS, 100 ng/mL and C3aR agonist, 25 μM) and extracellular ATP measured at 4 hours postactivation (top panel) and cell viability measured at 4 hours and 24 hours postactivation (bottom panel; data shown are the 24-hour time point). (E) C3aR-mediated extracellular ATP increase can be inhibited by CBX. Monocytes were activated as indicated in the presence or absence of 200 μM CBX and ATP efflux (top panel, at 4 hours postactivation) and IL-1β secretion (bottom panel, at 8 hours postactivation) assessed. Data shown in panels A-B,D-E represent mean ± SD (n = 3). Results shown in panel C are the mean values of data derived from 2 independent experiments with activation conditions performed in triplicate. *P < .05; **P < .005; ***P < .001 as determined using the paired t test with Bonferroni correction.
C3aR-mediated increase in IL-1β production is regulated via ATP cell efflux. (A-C) Monocyte treatment with oxATP, with apyrase or with a specific P2X7 antagonist abrogates LPS and LPS+C3a agonist-mediated IL-1β production. Monocytes were either left untreated or treated for 30 minutes with 300 μM oxATP (A), with 2.5 units/mL apyrase (B), or with 1 μM of the specific P2X7 antagonist AZ11645373 (C) before 24-hour activation with LPS and C3aR agonist (C3aRag) and subsequent assessment of IL-1β secretion. (D) C3aR activation increases extracellular ATP but does not affect cell viability. Freshly purified monocytes were activated as depicted (LPS, 100 ng/mL and C3aR agonist, 25 μM) and extracellular ATP measured at 4 hours postactivation (top panel) and cell viability measured at 4 hours and 24 hours postactivation (bottom panel; data shown are the 24-hour time point). (E) C3aR-mediated extracellular ATP increase can be inhibited by CBX. Monocytes were activated as indicated in the presence or absence of 200 μM CBX and ATP efflux (top panel, at 4 hours postactivation) and IL-1β secretion (bottom panel, at 8 hours postactivation) assessed. Data shown in panels A-B,D-E represent mean ± SD (n = 3). Results shown in panel C are the mean values of data derived from 2 independent experiments with activation conditions performed in triplicate. *P < .05; **P < .005; ***P < .001 as determined using the paired t test with Bonferroni correction.
C3a contributes to ATP efflux via ERK1/2 phosphorylation
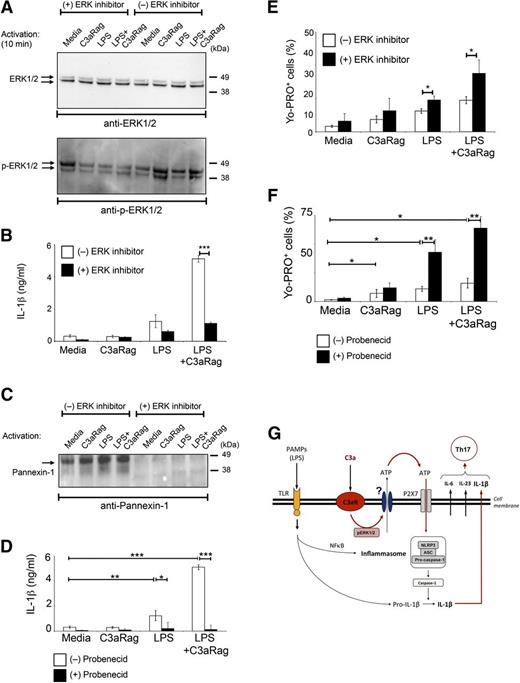
We next tried to identify the ATP efflux channel activated by C3aR engagement as well as a key signaling mediator triggered by the C3aR. C3aR activation leads to phosphorylation of the ERK1/2 in mesenchymal stem cells and DCs.33,34 Activation of monocytes in presence of the C3aR agonist also induced ERK1/2 phosphorylation and this event was prevented using a specific inhibitor of ERK1/2 phosphorylation (Figure 5A). Furthermore, activation of monocytes in the presence of ERK1/2 inhibition significantly reduced IL-1β secretion (Figure 5B). LPS, C3aR, and LPS+C3aR activation increased the expression of pannexin-1 channels in monocytes in an ERK1/2-dependent fashion (Figure 5C), while expression of connexin 43 was unaffected by any activation condition (data not shown). Although addition of the pannexin-1–specific inhibitor Probenecid abrogated activation-induced IL-1β production (Figure 5D), our unexpected observation that ERK1/2 inhibition and Probenecid addition leads to increased numbers of Yo-PRO–positive monocytes upon activation (Figure 5E-F) rather than the usually observed reduction in Yo-PRO–positive cells,35,36 suggests that the C3aR triggers ATP efflux via a pannexin-1–independent mechanism.
C3aR-mediated signal transduction in monocytes involves ERK1/2 phosphorylation. (A) C3aR stimulation induces ERK1/2 phosphorylation. Monocytes were either left in untreated media or preincubated 30 minutes in media with 1 μM MEK inhibitor (a reagent that prevents ERK1/2 phosphorylation) and then activated as indicated for 2, 4, 10, and 30 minutes. ERK1/2 phosphorylation was assessed in cell lysates by western blot analyses comparing nonphosphorylated ERK1/2 (ERK1/2) vs phosphorylated ERK1/2 (p-ERK1/2) (shown is the 10-minute time point, when ERK1/2 phosphorylation peaked). (B) Inhibition of ERK phosphorylation decreases IL-1β production. Monocytes were activated as described in panel A and IL-1β secretion measured at 18 hours postactivation. (C) Inhibition of ERK1/2 phosphorylation prevents pannexin-1 channel expression/opening upon monocyte activation. Experiments were performed as described in panel A and pannexin-1 channel expression was measured by western blot analysis at 8 hours postactivation. (D) Inhibition of pannexin-1 channel function impairs IL-1β production. Monocytes were activated as depicted in the presence or absence of 3 mM Probenecid and IL-1β secretion was measured 18 hours postactivation. (E-F) ERK1/2 or pannexin-1 channel inhibition results in reduced cellular efflux. Cells were activated as shown and retention of Yo-PRO dye measured 4 hours postactivation by FACS analysis. Results shown in panels A,C are representative of 2 separately performed experiments and results shown in panels B,D-F represent mean ± SD derived from 3 independent experiments (n = 3). *P < .05; **P < .001; ***P < .0005, determined by the paired t test with Bonferroni correction. (G) Model schematic of TLR and C3aR-mediated crosstalk leading to increased IL-1β production by human monocytes. TLR-mediated signals involving NF-κB translocation activate the NLRP3 inflammasome, which leads to subsequent caspase-1 activation and cleavage of inactive IL-1β into its active form. C3a generation during infection or inflammation engages the C3aR which induces an ERK1/2 phosphorylation-dependent increase in extracellular ATP availability. This in turn potentiates P2X7 signaling and caspase-1 activation cumulating in substantially increased IL-1β secretion. FACS, fluorescence-activated cell sorter.
C3aR-mediated signal transduction in monocytes involves ERK1/2 phosphorylation. (A) C3aR stimulation induces ERK1/2 phosphorylation. Monocytes were either left in untreated media or preincubated 30 minutes in media with 1 μM MEK inhibitor (a reagent that prevents ERK1/2 phosphorylation) and then activated as indicated for 2, 4, 10, and 30 minutes. ERK1/2 phosphorylation was assessed in cell lysates by western blot analyses comparing nonphosphorylated ERK1/2 (ERK1/2) vs phosphorylated ERK1/2 (p-ERK1/2) (shown is the 10-minute time point, when ERK1/2 phosphorylation peaked). (B) Inhibition of ERK phosphorylation decreases IL-1β production. Monocytes were activated as described in panel A and IL-1β secretion measured at 18 hours postactivation. (C) Inhibition of ERK1/2 phosphorylation prevents pannexin-1 channel expression/opening upon monocyte activation. Experiments were performed as described in panel A and pannexin-1 channel expression was measured by western blot analysis at 8 hours postactivation. (D) Inhibition of pannexin-1 channel function impairs IL-1β production. Monocytes were activated as depicted in the presence or absence of 3 mM Probenecid and IL-1β secretion was measured 18 hours postactivation. (E-F) ERK1/2 or pannexin-1 channel inhibition results in reduced cellular efflux. Cells were activated as shown and retention of Yo-PRO dye measured 4 hours postactivation by FACS analysis. Results shown in panels A,C are representative of 2 separately performed experiments and results shown in panels B,D-F represent mean ± SD derived from 3 independent experiments (n = 3). *P < .05; **P < .001; ***P < .0005, determined by the paired t test with Bonferroni correction. (G) Model schematic of TLR and C3aR-mediated crosstalk leading to increased IL-1β production by human monocytes. TLR-mediated signals involving NF-κB translocation activate the NLRP3 inflammasome, which leads to subsequent caspase-1 activation and cleavage of inactive IL-1β into its active form. C3a generation during infection or inflammation engages the C3aR which induces an ERK1/2 phosphorylation-dependent increase in extracellular ATP availability. This in turn potentiates P2X7 signaling and caspase-1 activation cumulating in substantially increased IL-1β secretion. FACS, fluorescence-activated cell sorter.
Taken together, our results support a model in which TLR-mediated and C3aR-mediated signals synergize for induction of IL-1β in macrophages and DCs and for increased IL-1β production in monocytes. TLR engagement involves nuclear factor–κB (NF-κB) translocation and assembly of the NLRP3 inflammasome leading to subsequent caspase-1 activation and generation of active IL-1β.37 C3a, which is generated at sites of inflammation, engages the C3aR and increases ATP efflux in an ERK1/2-dependent fashion via an as-yet unidentified channel or secretory system. Increased ATP availability in turn furthers P2X7 signaling which then potentiates NLRP3 inflammasome-driven pro-caspase-1 cleavage and IL-1β secretion (Figure 5G). This model is supported by the finding that C3aR-mediated signals also regulate IL-18 production, a cytokine that requires the same “ATP/P2X7/NLRP3/caspase-1” axis for its induction (supplemental Figure 7).
Discussion
Here, we further solidify the emerging axis between complement and the induction of effector T-cell responses and we suggest a novel pathway of C3aR-mediated signaling in monocytes, namely the increase of extracellular ATP efflux via ERK1/2-mediated signals upon LPS stimulation. It has been shown previously that C5a modulates Th17 responses. For example, TLR2 and C5aR activation synergize in mouse DCs and promote IL-12p70 production; however, C5aR-deficient splenic DCs produce increased amounts of IL-6 and IL-23 compared with C5aR-sufficient DCs when stimulated with the TLR ligand Pam3Cys.21 Furthermore, Lajoie and colleagues observed that C5a suppresses house dust mite-induced IL-23 production from bone marrow–derived DCs in a mouse model of asthma.38 In contrast, C5aR-deficient bone marrow–derived DCs are unable to produce IL-1β, IL-6, and IL-23 when activated with ovalbumin and LPS.39 Thus, the specific effects of C5a on IL-17–driving cytokine production in mouse models is dependent on the antigen-presenting cell type sensing the C5a signal and the TLR that is coengaged; a similar picture emerges for human antigen-presenting cells.40 Here, we show that C3aR engagement in human monocytes increases the release of TLR4-mediated cytokines, leading to enhanced Th17 responses. We further observed that C5aR engagement, without TLR coactivation, induces a strong IL-6 response in human monocytes. This is in line with a recent study demonstrating that Candida albicans induces IL-6 secretion in human peripheral blood mononuclear cells in a C5a-dependent fashion.41 Together, these data suggest that both C3a and C5a participate in the generation and modulation of Th17 responses42 by regulating IL-6 and IL-1β. Most studies assessing the role of C3a in inflammatory settings have been performed using C3aR-deficient mice as individuals with C3aR deficiencies have not been described. Interestingly, depending on the particular disease model for which these animals were used, lack of C3aR-mediated signals could either lead to a decrease43 or an increase in IL-1β.44,45 This suggests that, similar to C5a, the effect of C3a on IL-1β induction depends on the nature of the response-evoking agent, the site of infection or DAMP occurrence and the particular composition of immune cells migrating into the site of the insult. In line with this, C3aR activation did not alter nigericin-induced IL-1β production in monocytes (supplemental Figure 8).
TLRs and the complement system share a common initiation signal characterized by PAMPs, which is vital to the instruction of adaptive immune responses.19,20,46 Assessing the importance of C3a-driven IL-1β production in monocytes and its impact on Th17 generation in a small animal model of infection is hampered by the fact that monocyte subpopulations and functions differ substantially between mice and humans, questioning the transferability of results between the 2 species.47 However, TLRs and complement evolved to also recognize noxious signals derived from altered self, such as injured tissue or stressed cells,18 a scenario characterizing a wide range of autoimmune diseases as well as graft rejection.18 Models assessing the contribution of immune cells in transplantation have the advantage that the location and time point of the “insult” are known and that subsequent immune reactions can be monitored in part by analyses of blood and biopsy samples. Our data obtained here using such biopsy samples suggest (but can, of course, not unequivocally prove) the following scenario: IRI-mediated cellular stress induces local C3a (and C5a) generation and release of DAMP-associated ligands for TLR4 (such as high mobility group box 1 protein [HMGB1]) within the donor organ.48,49 The anaphylatoxin-rich environment instructs IL-1β and IL-6 secretion in monocytes migrating into the site of inflammation while IL-23 coproduction in monocytes is TLR-driven. T cells arriving with the second wave of infiltrating immune-competent cells find a Th17-conducive environment and develop in large proportions into this effector phenotype, further contributing to tissue destruction. High local anaphylatoxin concentrations are involved in Th1 induction and are a hallmark of several autoimmune diseases including rheumatoid arthritis.21,50,51 Thus, although here demonstrated on inflamed rejecting kidney grafts, the local actions of complement and TLRs designed to induce protective Th1 and/or Th17 responses upon pathogen sensing, likely also perpetuate unwanted Th1 and Th17 responses triggered via DAMPs (for example dying cells or uncontrolled exposure of intracellular antigens)52,53 in autoimmune or ischemic settings.
Although few studies have begun to investigate C3aR-mediated signaling in human macrophages and DCs,19,32 this is the first study to assess C3aR-mediated signals in human monocytes. It is becoming increasingly apparent that monocytes are not only critical in the initial phases of pathogen clearance, but also in autoimmune settings. Our observations here, showing that monocytes are the prime source of proinflammatory cytokines, are in line with this notion. Characterization of molecular changes in monocytes associated with specific inflammatory signals, therefore, may help improve therapeutic targeting to stimulate or dampen downstream events.53 We have shown here that C3aR-mediated enhancement of IL-1β production is regulated by ERK1/2 activation and subsequent increased ATP efflux. Interestingly, although C3aR activation seems to induce pannexin-1 expression, and C3aR-mediated effects are sensitive to Carbenoxolon and Probenecid treatment, our data indicate that another, yet to be identified, channel15-17 or pathway54 is involved in the C3aR-driven ATP efflux. This aligns with the observation that macrophages from pannexin-1–deficient mice still produce IL-1β.55
Another important outcome of this study is our observation that human macrophages (and DCs), at least in vitro, require both TLR and C3aR activation for IL-1β production. This is in line with a recent report demonstrating that although IL-1β production by monocytes required a one-time TLR stimulation, macrophages and DCs required a second stimulation with ATP in addition to TLR-mediated signals for IL-1β release.12 The authors also observed that macrophages, as opposed to monocytes, are not capable of ATP release and required exogenous ATP for P2X7 activation. Our finding that C3aR expression is dramatically decreased on macrophages and that C3aR agonist addition does not induce ATP release in these cells (supplemental Figure 9), suggests that they may indeed depend more on exogenous ATP. This would fit into the concept that protective innate immune responses need to be tightly controlled to prevent exuberant tissue pathology and restore immune homeostasis.18 Tissue-resident monocytes and monocytes infiltrating injured tissue are danger sentinels and respond quickly and with a low signal threshold to PAMP/DAMP presence with the secretion of high amounts of proinflammatory cytokines.47,56 The microenvironment at tissue sites then induces a phenotypic change of monocytes into more macrophage-like cells characterized by a substantial decrease in IL-1β and IL-6 production.47 In addition, macrophages at inflammatory sites in particular are a rather diverse population. Depending on incoming cytokine signals, they assume an M1 proinflammatory phenotype, further aiding in pathogen clearance, or become phenotypically M2 macrophages, which are important for the control of effector T-cell responses and tissue repair.56 It is possible that these latter cells may resist TLR and complement-mediated signals and can therefore restore tissue and immune homeostasis even in settings of still-heightened immune activation.
Thus, this study further underpins our growing understanding that complement receptors participate in a much wider range of physiological processes than previously anticipated57 and that the net outcome of complement activation on immune responses depends heavily on additional incoming cues provided by other danger sensing systems such as TLRs as well as the nature of the signal-receiving cell.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John Harris from the NIKON Imaging Centre at King’s College London for his help in the generation of the confocal microscopy images.
This work was supported by a grant from Kidney Research UK to E.A. (TF9.2009), an MRC Research Grant awarded to C.K. (G1002165), the Medical Research Council Centre for Transplantation, Guy’s Hospital, King’s College, and the Department of Health, National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. J.K. was supported by the German Research Foundation (GRK1727 TP8 and SFB/TR22 A21) and H.T.C. received support from the Imperial College Healthcare NHS Trust Biomedical Research Centre.
Authorship
Contribution: E.A. designed and performed the majority of the experiments, interpreted the data, and wrote the manuscript; G.L.F. performed a large proportion of experiments, helped with data interpretation and edited the manuscript; H.Y. designed and performed the immunohistology experiments; E.P. designed the Th17 experiments and helped with the data interpretation; S.S.S. edited the manuscript; J.K. designed experiments, assisted with data interpretation and edited the manuscript; H.T.C. provided the histology samples, designed experiments, discussed data, and edited the manuscript; and C.K. perceived and designed the study, performed experiments, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudia Kemper, MRC Centre for Transplantation, King’s College London, Guy’s Hospital 5th Floor, Tower Wing, Great Maze Pond, London SE1 9RT, United Kingdom; e-mail: claudia.kemper@kcl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal