To the editor:
The NCI common toxicity criteria1 (CTC) are a safety feature that guide choice of dose in clinical trials, but for asymptomatic toxicities involving bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine, these criteria are not based on empiric data. One means to do this is to relate toxicity criteria for these laboratory tests to treatment-associated mortality (TAM). For this purpose, we examined 714 courses of chemotherapy given to 511 patients with newly diagnosed or relapsed/refractory acute myeloid leukemia between 2002 and 2012. TAM was defined as death within 28 days.2 Maximal values for creatinine, total bilirubin, AST, and ALT in the first 28 days after initiation of treatment were related to TAM using the CTC. To approximate patients in an early phase trial, we excluded patients with acute promyleocytic leukemia, those who previously had a stem cell transplant, and those with initial values of either creatinine or bilirubin >2. Many patients received experimental treatments, with only 56% receiving cytarabine and only 40% anthracycline. TAM occurred in 39 patients (8%, 5.6% of 714 courses). Here and in Figure 1, we list significant (P < .05) associations between the CTC and TAM.
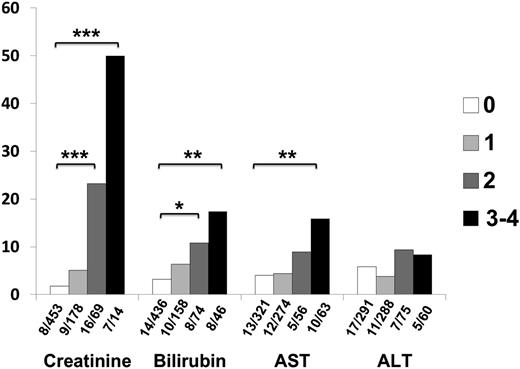
The percentage of chemotherapy treatments resulting in death by 28 days for a given toxicity grade. For each toxicity grade, the number of patients who died within 28 days of initiating treatment divided by the number of patients with that toxicity grade is shown below the graph. P values were calculated by Fisher exact test, and divided by one third to account for multiple comparisons for each toxicity. *P < .05, **P < .005, ***P < .0005.
The percentage of chemotherapy treatments resulting in death by 28 days for a given toxicity grade. For each toxicity grade, the number of patients who died within 28 days of initiating treatment divided by the number of patients with that toxicity grade is shown below the graph. P values were calculated by Fisher exact test, and divided by one third to account for multiple comparisons for each toxicity. *P < .05, **P < .005, ***P < .0005.
Elevated creatinine was closely correlated to TAM. TAM was only 1.8% (8/453) in courses where the creatinine remained normal, but grade 2 toxicity was associated with an increase to 23% (16/69, P = .002), whereas grade 3 or 4 toxicity was associated with 50% (7/14, P < .0001) TAM. Bilirubin showed less correlation with TAM. With no bilirubin toxicity, TAM was 3.2% (14/436) vs 11% (8/74, P = .024) with grade 2 toxicity, and 17% (8/46, P = .0012) with grade 3 or 4 toxicity. For AST, TAM rates were 4% (13/321) for no toxicity and 15% (10/63, P = .0036) for grade ≥3 toxicity. ALT was not significantly correlated with TAM, even at grade ≥3 (8.3%, 5/60 vs 5.8% at grade 0, 17/291; P = .55). To reduce the influence of patients who had similar toxicities on successive courses, we also analyzed results by patient rather than course. Results were qualitatively similar, but grade 2 bilirubin toxicity and any grade of AST toxicity were not statistically significantly associated with mortality.
We acknowledge that these toxicities were associated with, rather than a cause of, death. The number of deaths was insufficient to examine the effect of other covariates (eg, cytotoxic drugs vs targeted therapies used alone, age) on TAM. However, decisions regarding dose changes using the current system are also based purely on association, and, likewise, do not recognize covariates other than dose.3 We recognize that toxicities are important not only as they relate to death. Nonetheless, we feel that the association of some asymptomatic toxicities, but not others, with death in this population is meaningful. We suggest the use of empirical data, specifically associations between degree of toxicity for creatinine, bilirubin, AST, and ALT, as well as subsequent TAM, to help guide decisions about doses in early trials in acute myeloid leukemia.
This work was presented in poster form at the American Society of Hematology meeting, December 2012, Atlanta, GA.
Authorship
Acknowledgments: Informed approval for this research was obtained from the Fred Hutchinson Cancer Research Center's Institutional Review Board. Informed consent for the relevant studies was obtained according to the Declaration of Helsinki.
Contribution: J.R.V and E.E. designed the research; J.R.V. and V.S. performed the research; J.R.V., P.S.B., J.M.P., F.R.A., and E.E. analyzed the data; and J.R.V., P.S.B., F.R.A., and E.E. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elihu Estey, Fred Hutchinson Cancer Research Center, Mailstop 3200, 1100 Fairview Ave N., PO Box 19024, Seattle, WA 98109; e-mail: eestey@seattlecca.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal