Key Points
Gain-of-function Lyn mice develop hemolytic anemia with acanthocyte red blood cells and display compensatory extramedullary erythropoiesis.
Hyperactive Lyn notably alters Epo receptor signaling, particularly an Akt-FoxO3 pathway, enhancing viability and delaying differentiation.
Abstract
Lyn is involved in erythropoietin (Epo)-receptor signaling and erythroid homeostasis. Downstream pathways influenced following Lyn activation and their significance to erythropoiesis remain unclear. To address this, we assessed a gain-of-function Lyn mutation (Lynup/up) on erythropoiesis and Epo receptor signaling. Adult Lynup/up mice were anemic, with dysmorphic red cells (spherocyte-like, acanthocytes) in their circulation, indicative of hemolytic anemia and resembling the human disorder chorea acanthocytosis. Heterozygous Lyn+/up mice became increasingly anemic with age, indicating that the mutation was dominant. In an attempt to overcome this anemia, extramedullary erythropoiesis was activated. As the mice aged, the levels of different immature erythroid populations changed, indicating compensatory mechanisms to produce more erythrocytes were dynamic. Changes in Epo signaling were observed in Lyn+/up erythroid cell lines and primary CD71+Lynup/up erythroblasts, including significant alterations to the phosphorylation of Lyn, the Epo receptor, Janus kinase 2, Signal Transducer and Action of Transcription-5, GRB2-associated-binding protein-2, Akt, and Forkhead box O3. As a consequence of altered Lyn signaling, Lyn+/up cells remained viable in the absence of Epo but displayed delayed Epo-induced differentiation. These data demonstrate that Lyn gene dosage and activity are critical for normal erythropoiesis; constitutively active Lyn alters Epo signaling, which in turn produces erythroid defects.
Introduction
The primary regulator of committed erythroid progenitors is erythropoietin (Epo) through its engagement of the Epo receptor and subsequent activation of intracellular signaling cascades, including the Janus kinase/signal transducer and activator of transcription (JAK/STAT), Rat sarcoma/rapidly accelerated fibrosarcoma/mitogen activated protein (ras/raf/MAP)-kinase, and Phosphatidylinositide 3 (PI3) kinase/Akt pathways.1-4 JAK2 is recognized as the kinase involved in initiation of Epo receptor signaling,1 whereas Lyn, a member of the Src family of tyrosine kinases (SFKs), has been implicated as a key secondary kinase.5-10
In vitro studies established a role for Lyn in Epo receptor signaling6,11 and demonstrated that Epo signaling in immortalized J2E-derived cells appeared identical to primary erythroid cells.6,9,11 Subsequent studies using Lyn−/− mice consolidated the importance of this SFK for early erythroid cell expansion and late-stage maturation.8-10 Removal, or inhibition, of Lyn attenuates the ability of erythroid cells to differentiate in response to Epo.6,8-10
On Epo receptor ligation, Lyn is activated6,11 and intersects numerous downstream signaling events, including phosphorylation of STAT5.6,7 Reduced Lyn in erythroid cell lines diminishes erythroid transcription factor (GATA-1 [GATA-binding factor 1], EKLF [Erythroid Kruppel-like Factor]) levels and reduces their ability to differentiate; conversely, transient overexpression of Lyn enhances differentiation.6,11 In addition, Lyn stimulates pathways that lead to down-regulation of Epo receptor signaling.12,13
Mice deficient in Epo, Epo receptor, and JAK2 fail to develop a definitive erythroid compartment.14-16 Although definitive erythropoiesis occurs in Lyn−/− mice, an underlying erythroid defect results in activation of compensatory stress erythropoiesis.8-10 Although the absence of Lyn clearly perturbs erythropoiesis and affects Epo receptor signaling,9,10 the pathways influenced on Lyn activation and their biological roles remain unclear. To address this important question, we examined knock-in mice expressing a constitutively active form of Lyn (Lynup/up), such that constitutively active Lyn would be expressed in a temporally and spatially appropriate manner.13
In this article, we demonstrate that Lynup/up mice displayed a hemolytic anemia and had markedly elevated erythroid progenitors and precursors primarily in spleen, indicating active extramedullary erythropoiesis. Importantly, constitutively active Lyn had a major impact on Epo receptor signaling, most notably affecting the JAK2-STAT5, GRB2-associated-binding protein 2 (GAB2), and Akt–forkhead box O3 (FoxO3) pathways, suggesting that regulation of Lyn is crucial for normal erythropoiesis. Failure to control Lyn activity, as exemplified by Lyn deficiency or its overactivity, interferes with Epo receptor signaling and is deleterious for erythroid homeostasis.
Materials and methods
Mice, cell morphology, and anemia induction
Lyn−/−, Lynup/up, Lyn+/up, and Lyn+/+ mice,13,17 on a C57BL/6 background, were analyzed at days 12.5 to 13.5 of embryonic development, as 8- to 15-week-old adults, and as aged (70-85 weeks old) animals. The Lynup allele is a knock-in activating point mutation of Tyr-to-Phe at residue 508 (the C-terminal regulatory Tyr) of the Lyn gene, generating a constitutively active kinase. All experiments were performed in accordance with National Health and Medical Research Council guidelines for animal experimentation, with approval from the Animal Ethics Committees of the Baker IDI Heart and Diabetes Institute (Melbourne, Australia), the Animal Resource Centre, (Murdoch, Australia), and the Royal Perth Hospital (Perth, Australia). Heparinized pediatric tubes were used for blood collection and blood cell parameter determination on an Advia 120 (Siemens, Deerfield, IL). Blood smears, bone marrow, and fetal liver and splenic cell morphology were examined microscopically following staining.18 Anemia was induced by injection of phenylhydrazine (PHZ; 60 mg/kg body weight) on 2 consecutive days, followed by blood and erythroid cell analysis 3 and 5 days after initial PHZ injection as previously described.19 Primary CD71+ spleen erythroblasts were isolated from day 5 PHZ-treated mice using fluorescein isothiocyanate-CD71 (BD Biosciences, San Jose, CA) and EasySep magnetic FITC Selection Kit (StemCell Technologies, Vancouver, BC, Canada) essentially as previously described.10
Erythroid progenitor assays and flow cytometry
Single cell suspensions of bone marrow and spleen were prepared and assayed for erythroid burst-forming units (BFU-E) and colony-forming units (CFU-E) using methylcellulose cultures, as described.9 Flow cytometry was used to assess erythroid populations in bone marrow and spleen as detailed previously9,10 using a FACS Aria II flow cytometer (Beckman-Coulter, Palo Alto, CA).
Erythroid cell lines
Immortalized erythroid cell lines were generated by exposing fetal liver progenitors (day E12.5 embryos) to J2 retrovirus as described.20 Individual clones (12 per genotype) were isolated and characterized for viability and hemoglobin production as described.9 Cells were maintained in Iscove's modified Dulbecco's medium (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum. Short-term Epo inductions (5 units/mL, Epoetin alfa; Janssen-Cilag) were carried out on cells after 2-hour serum starvation. Differentiation experiments were performed on cells cultured in thyroid hormone–depleted fetal bovine serum in the presence or absence of Epo (5 units/mL).21
Immunoblotting and immunoprecipitation
Cells were lysed in raft buffer (150 mm NaCl, 1% octylphenoxypolyethoxyethanol (IGEPAL CA-630) [Sigma-Aldrich, St. Louis, MO], 0.5% n-dodecyl-β-d-maltoside [Sigma-Aldrich], 0.2% octyl-d-glucoside [Sigma-Aldrich], 20 mm Tris, pH 8.0, 1× Complete protease inhibitor cocktail [Roche, Mannheim, Germany], 2 mM benzamidine, 2 mM vanadate, 1 mM EDTA, 1 mM EGTA, and 10 mM β-glycerol phosphate). Signaling studies were undertaken by immunoprecipitation and immunoblotting as previously described using antibodies as described in the supplemental Materials and methods on the Blood website.21
Results
Gain-of-function Lyn induces hemolytic anemia
Young adult and aged Lynup/up mice displayed overt signs of anemia, with hematocrits, hemoglobin content, and circulating red blood cells all significantly reduced (Table 1). Interestingly, young Lyn+/up mice displayed an intermediate phenotype; however, with age, they developed more severe symptoms of anemia, and the decrease in all red cell parameters became statistically significant (Table 1). Blood smears revealed that Lynup/up mice contained significant numbers of abnormal red blood cells in circulation (Figure 1A). The presence of numerous circulating acanthocytes (Lyn+/+, 0.1 ± 0.0%; Lynup/up, 14 ± 9.3%; P = .002) and spherocyte-like cells (Lyn+/+, 0.1 ± 0.0%; Lynup/up, 10.3 ± 12.9%; P = .04) suggested the animals had a hemolytic anemia. These data demonstrate that expression of either 1 (Lyn+/up) or 2 (Lynup/up) hyperactive Lyn alleles leads to anemia and red blood cell abnormalities.
Red blood cell parameters of Lyn+/+, Lyn+/up, and Lynup/up mice
| Age (wk) . | Genotype . | HCT (%) . | HGB (g/L) . | RBC (×1012/L) . |
|---|---|---|---|---|
| 10-15 | Lyn+/+ | 50.1 ± 0.04 | 126 ± 10.4 | 8.5 ± 0.87 |
| 10-15 | Lyn+/up | 47.0 ± 0.02* | 118 ± 6.5ns | 7.9 ± 0.37ns |
| 10-15 | Lynup/up | 38.0 ± 0.03* | 102 ± 7.8* | 6.5 ± 0.52* |
| 70-85 | Lyn+/+ | 47.6 ± 0.02 | 126 ± 6.8 | 9.0 ± 0.49 |
| 70-85 | Lyn+/up | 41.9 ± 0.02* | 114 ± 5.9* | 7.8 ± 0.49* |
| 70-85 | Lynup/up | 41.2 ± 0.13* | 108 ± 10.3* | 7.4 ± 1.62* |
| Age (wk) . | Genotype . | HCT (%) . | HGB (g/L) . | RBC (×1012/L) . |
|---|---|---|---|---|
| 10-15 | Lyn+/+ | 50.1 ± 0.04 | 126 ± 10.4 | 8.5 ± 0.87 |
| 10-15 | Lyn+/up | 47.0 ± 0.02* | 118 ± 6.5ns | 7.9 ± 0.37ns |
| 10-15 | Lynup/up | 38.0 ± 0.03* | 102 ± 7.8* | 6.5 ± 0.52* |
| 70-85 | Lyn+/+ | 47.6 ± 0.02 | 126 ± 6.8 | 9.0 ± 0.49 |
| 70-85 | Lyn+/up | 41.9 ± 0.02* | 114 ± 5.9* | 7.8 ± 0.49* |
| 70-85 | Lynup/up | 41.2 ± 0.13* | 108 ± 10.3* | 7.4 ± 1.62* |
HCT, hematocrit; HGB, hemoglobin; ns, not significant; RBC, red blood cell.
P ≤ .05.
Alterations to erythroid tissues in Lynup/up mice. (A) Peripheral blood from adult Lynup/up mice contains acanthocytes and spherocyte-like erythrocytes. Blood smears from adult Lyn+/+ and Lynup/up mice stained with Wright-Giemsa (black arrowhead = acanthocyte, white arrowhead = spherocyte-like, scale bar = 10 µm). (B) The bone marrow of adult Lynup/up mice displays altered ratios of erythroid and granulocytic cells compared with Lyn+/+ animals. Cytopreps of single cell suspensions of bone marrow from adult Lyn+/+ and Lynup/up mice were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = erythroblasts, scale bar = 20 µm). (C) Lynup/up spleens contain normal and abnormal erythroid/blast cells. Cytopreps of single cell suspensions of spleen from adult Lyn+/+ and Lynup/up mice were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = erythroblasts, black arrowhead = abnormal erythroblasts, yellow arrowhead = blasts, scale bar = 20 µm). (D) Lynup/up fetal livers (E12.5) contain significant numbers of definitive (enucleated) erythrocytes compared with Lyn+/+ animals. Cytopreps of single cell suspensions of fetal liver (E12.5) of Lyn+/+ and Lynup/up embryos were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = definitive [enucleated] erythrocytes, scale bar = 20 µm).
Alterations to erythroid tissues in Lynup/up mice. (A) Peripheral blood from adult Lynup/up mice contains acanthocytes and spherocyte-like erythrocytes. Blood smears from adult Lyn+/+ and Lynup/up mice stained with Wright-Giemsa (black arrowhead = acanthocyte, white arrowhead = spherocyte-like, scale bar = 10 µm). (B) The bone marrow of adult Lynup/up mice displays altered ratios of erythroid and granulocytic cells compared with Lyn+/+ animals. Cytopreps of single cell suspensions of bone marrow from adult Lyn+/+ and Lynup/up mice were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = erythroblasts, scale bar = 20 µm). (C) Lynup/up spleens contain normal and abnormal erythroid/blast cells. Cytopreps of single cell suspensions of spleen from adult Lyn+/+ and Lynup/up mice were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = erythroblasts, black arrowhead = abnormal erythroblasts, yellow arrowhead = blasts, scale bar = 20 µm). (D) Lynup/up fetal livers (E12.5) contain significant numbers of definitive (enucleated) erythrocytes compared with Lyn+/+ animals. Cytopreps of single cell suspensions of fetal liver (E12.5) of Lyn+/+ and Lynup/up embryos were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = definitive [enucleated] erythrocytes, scale bar = 20 µm).
Altered erythropoiesis in Lyn+/up and Lynup/up mice
The bone marrow of Lynup/up mice displayed an altered ratio of erythroid to granulocytic precursors, with proportionally more granulocytic precursors (meyloid:erythroid ratio: Lyn+/+, 4:1 ± 2.0; Lynup/up, 7:1 ± 1.9; P = .03; Figure 1B), reflecting their elevated peripheral neutrophil counts.13 Lynup/up spleen cell preparations showed reduced lymphocytes (lymphocytes: Lyn+/+, 81 ± 6%; Lynup/up, 31 ± 7%; P < .01) and increased erythroid precursors, blast/progenitor-like cells, and abnormal large poly/ortho-chromatic-erythroid cells (Figure 1C). Interestingly, the fetal livers of 12.5-day-old Lynup/up embryos displayed numerous small enucleated (definitive) erythrocytes, whereas control animals possessed mostly large nucleated (primitive) erythrocytes that are normally found at this embryonic stage (enucleated erythrocytes: Lyn+/+, 5.6 ± 9.7%; Lynup/up, 47.8 ± 18.7%; P < .001; Figure 1D). These morphological analyses show a significant effect of elevated Lyn activity on the erythroid compartment during embryonic development and in adult tissues.
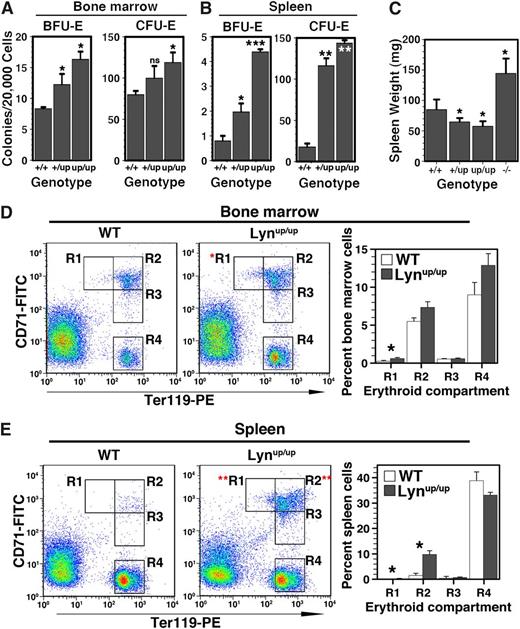
Progenitors in the bone marrow of young adult Lyn+/up and Lynup/up mice were raised: BFU-E numbers were significantly higher, whereas CFU-E were elevated by ∼50% in Lynup/up and ∼20% in Lyn+/up mice (Figure 2A). In spleens, changes were even more dramatic, with BFU-E elevated 2-fold in Lyn+/up and >5-fold in Lynup/up, whereas CFU-E was increased >10-fold (Figure 2B). The significant extramedullary erythropoiesis was not accompanied by splenomegaly, unlike in Lyn−/− mice9,10 (Figure 2C); in fact, the spleens were statistically smaller in Lyn+/up and Lynup/up mice, due to a major B-cell deficit.22 Taking into account the reduced cellularity of Lyn+/up and Lynup/up spleens, absolute numbers of BFU-E and CFU-E were still markedly elevated four- and eightfold, respectively.
Elevated bone marrow and spleen erythropoiesis in Lyn+/up and Lynup/up mice. (A) Bone marrow BFU-E and CFU-E numbers are increased in Lyn+/up and Lynup/up adult mice. Erythroid colony assays (BFU-E and CFU-E) of bone marrow from Lyn+/+, Lyn+/up, and Lynup/up adult mice (8 weeks) (n = 4 in 2 independent experiments, *P < .05, ns, not significant). (B) Extramedullary erythropoiesis in spleen of Lyn+/up and Lynup/up adult mice. Erythroid colony assays (BFU-E and CFU-E) of spleens in Lyn+/+, Lyn+/up, and Lynup/up adult mice (8 weeks) (n = 4 in 2 independent experiments, *P < .05, **P < .001, ***P < .001). (C) Lynup/up and Lyn+/up adult mice display spleen reduction compared with controls and in contrasts to Lyn−/− animals that show splenomegaly. Spleen wet weight of adult mice (12-15 weeks) of the indicated Lyn genotypes (n > 6, *P < .05). (D) Comparison of maturing bone marrow erythroid cells from Lynup/up and Lyn+/+ adult mice. Representative flow cytometric analysis of bone marrow cells from Lyn+/+ (WT) and Lynup/up mice (12-15 weeks) using anti-CD71 and anti-Ter119 and enumeration of the indicated erythroid subsets (R1, R2, R3, R4; n > 3, *P < .05).49 (E) Elevated maturing erythroid cells in the spleen of Lynup/up adult mice. Representative flow cytometric analysis of spleen cells from Lyn+/+ (WT) and Lynup/up mice (12-15 weeks) using anti-CD71 and anti-Ter119 and enumeration as in D.
Elevated bone marrow and spleen erythropoiesis in Lyn+/up and Lynup/up mice. (A) Bone marrow BFU-E and CFU-E numbers are increased in Lyn+/up and Lynup/up adult mice. Erythroid colony assays (BFU-E and CFU-E) of bone marrow from Lyn+/+, Lyn+/up, and Lynup/up adult mice (8 weeks) (n = 4 in 2 independent experiments, *P < .05, ns, not significant). (B) Extramedullary erythropoiesis in spleen of Lyn+/up and Lynup/up adult mice. Erythroid colony assays (BFU-E and CFU-E) of spleens in Lyn+/+, Lyn+/up, and Lynup/up adult mice (8 weeks) (n = 4 in 2 independent experiments, *P < .05, **P < .001, ***P < .001). (C) Lynup/up and Lyn+/up adult mice display spleen reduction compared with controls and in contrasts to Lyn−/− animals that show splenomegaly. Spleen wet weight of adult mice (12-15 weeks) of the indicated Lyn genotypes (n > 6, *P < .05). (D) Comparison of maturing bone marrow erythroid cells from Lynup/up and Lyn+/+ adult mice. Representative flow cytometric analysis of bone marrow cells from Lyn+/+ (WT) and Lynup/up mice (12-15 weeks) using anti-CD71 and anti-Ter119 and enumeration of the indicated erythroid subsets (R1, R2, R3, R4; n > 3, *P < .05).49 (E) Elevated maturing erythroid cells in the spleen of Lynup/up adult mice. Representative flow cytometric analysis of spleen cells from Lyn+/+ (WT) and Lynup/up mice (12-15 weeks) using anti-CD71 and anti-Ter119 and enumeration as in D.
The maturation status of the erythroid compartment was then investigated using flow cytometry. An elevation in the most immature R1 (Lyn+/+, 0.12 ± 0.06%; Lynup/up, 0.76 ± 0.05%; P < .05) precursor population (CD71high Ter119low) was observed in the bone marrow of young adult Lynup/up mice (Figure 2D), commensurate with the rise in BFU-E and CFU-E (Figure 2A). In the spleens of Lynup/up mice, both R1 (Lyn+/+, 0.06 ± 0.05%; Lynup/up, 1.01 ± 0.12%; P < .01) and R2 (Lyn+/+, 1.96 ± 0.42%; Lynup/up, 9.81 ± 1.30%; P < .01) (CD71high/Ter119high) populations were markedly elevated (Figure 2E), consistent with the increase in splenic progenitors (Figure 2B). Lyn+/up mice also displayed a similar increase in R1 and R2 precursors (supplemental Figure 1). Together, these data suggest that hyperactive Lyn induces a red cell membrane defect causing hemolysis and anemia, leading to activation of extramedullary erythropoiesis to maintain homeostasis.
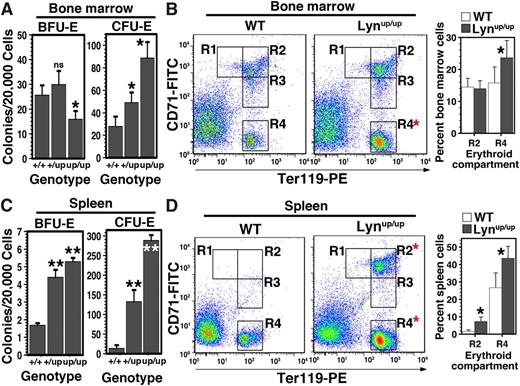
Erythroid progenitors and precursors were then examined in older mice. In contrast to young adult mice, numbers of bone marrow BFU-E were not significantly different in Lyn+/up but were decreased by ∼30% in Lynup/up, suggesting bone marrow exhaustion with age in Lynup/up mice (Figure 3A). However, CFU-E numbers remained elevated for both Lyn+/up and Lynup/up, indicating that later-stage expansion capacity persists (Figure 3A). A change in the precursor profile was also detected with a marked increase in the most mature R4 (CD71low/Ter119high) population (Figure 3B). These observations indicate that changes have occurred in the erythroid compartment with age in an attempt to maintain red cell levels.
Altered bone marrow erythropoiesis and elevated spleen erythropoiesis in aged Lyn+/up and Lynup/up mice. (A) Bone marrow BFU-E are reduced, whereas CFU-E numbers are increased in aged Lyn+/up and Lynup/up mice. Erythroid colony assays (BFU-E and CFU-E) of Lyn+/+, Lyn+/up, and Lynup/up bone marrow from mice 70 to 85 weeks of age (n ≥ 3, *P < .05, ns, not significant). (B) Comparison of maturing bone marrow erythroid cells from aged Lynup/up and Lyn+/+ mice. Representative flow cytometric analysis of Lyn+/+ (WT) and Lynup/up bone marrow cells of mice 70 to 85 weeks of age and enumerated as in Figure 2D. (C) Extramedullary erythropoiesis in spleen of aged Lyn+/up and Lynup/up mice. Erythroid colony assays (BFU-E and CFU-E) of Lyn+/+, Lyn+/up, and Lynup/up spleens from mice 70 to 85 weeks of age (n ≥ 3, **P < .01). (D) Elevated maturing erythroid cells in the spleen of aged Lynup/up mice. Representative flow cytometric analysis of spleen cells of Lyn+/+ (WT) and Lynup/up mice 70 to 85 weeks of age and enumerated as in B. Mean ± standard deviation is shown. Statistically significant (2-way ANOVA) differences are indicated (*P < .05).
Altered bone marrow erythropoiesis and elevated spleen erythropoiesis in aged Lyn+/up and Lynup/up mice. (A) Bone marrow BFU-E are reduced, whereas CFU-E numbers are increased in aged Lyn+/up and Lynup/up mice. Erythroid colony assays (BFU-E and CFU-E) of Lyn+/+, Lyn+/up, and Lynup/up bone marrow from mice 70 to 85 weeks of age (n ≥ 3, *P < .05, ns, not significant). (B) Comparison of maturing bone marrow erythroid cells from aged Lynup/up and Lyn+/+ mice. Representative flow cytometric analysis of Lyn+/+ (WT) and Lynup/up bone marrow cells of mice 70 to 85 weeks of age and enumerated as in Figure 2D. (C) Extramedullary erythropoiesis in spleen of aged Lyn+/up and Lynup/up mice. Erythroid colony assays (BFU-E and CFU-E) of Lyn+/+, Lyn+/up, and Lynup/up spleens from mice 70 to 85 weeks of age (n ≥ 3, **P < .01). (D) Elevated maturing erythroid cells in the spleen of aged Lynup/up mice. Representative flow cytometric analysis of spleen cells of Lyn+/+ (WT) and Lynup/up mice 70 to 85 weeks of age and enumerated as in B. Mean ± standard deviation is shown. Statistically significant (2-way ANOVA) differences are indicated (*P < .05).
Extramedullary erythropoiesis was also a feature of older Lyn+/up and Lynup/up mice, and splenic progenitors remained markedly elevated: BFU-E levels were raised 3-fold, whereas CFU-E were either 7- (Lyn+/up) or 15-fold (Lynup/up) higher (Figure 3C). Although the R2 population remained high in spleens of aged Lynup/up mice, an increase in the R4 population was also observed (Figure 3D). These data suggest that compensatory mechanisms to alleviate the anemia caused by hyperactive Lyn are dynamic and that erythroid progenitor and precursor components alter with age in an attempt to produce more erythrocytes.
Altered response to anemia by Lyn+/up mice
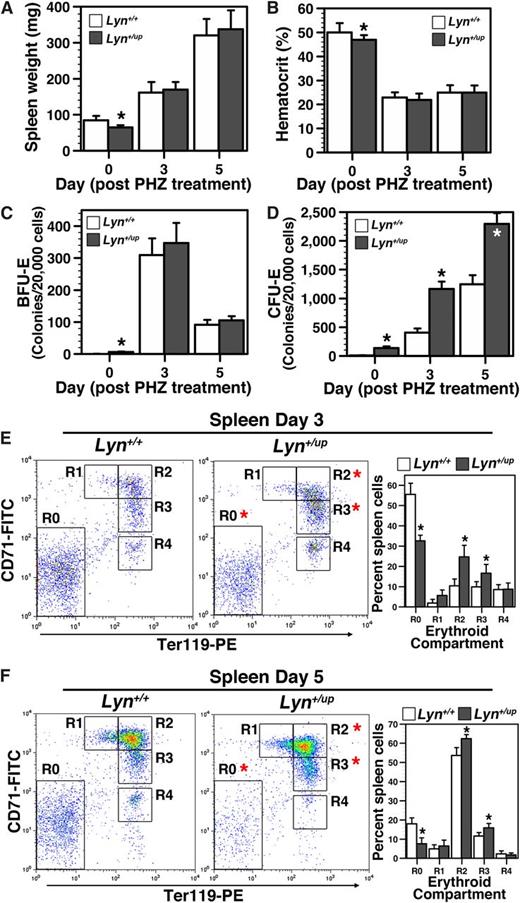
As Lyn+/up mice display a mild erythroid phenotype, we speculated that stressing their erythroid compartment might reveal more significant underlying perturbations. Accordingly, the ability of Lyn+/up mice to respond to acute anemia induced by PHZ was examined. Both control and Lyn+/up mice displayed comparable hematocrit reduction at 3 and 5 days after PHZ treatment (Figure 4A-B). Although no differences in BFU-E recovery were detected between control and Lyn+/up mice (Figure 4C), the CFU-E expansion at both days 3 and 5 after PHZ treatment was significantly elevated in Lyn+/up mice (Figure 4D). Flow cytometric analyses of erythroid cells 3 and 5 days after PHZ treatment also revealed significant differences in the R0 and maturing R2 and R3 populations (Figure 4E-F). Together, these data show that following acute anemic insult, Lyn+/up mice display an altered erythroid expansion response to recover their hematocrit.
Altered response to chemically induced anemia in Lyn+/up adult mice. (A) Lyn+/up mice respond the same as control animals to PHZ treatment induced splenomegaly. Spleen wet weight of Lyn+/up and Lyn+/+ adult mice (12-15 weeks) at 0, 3, and 5 days after PHZ treatment (n > 6, *P < .05). (B) Lyn+/up hematocrits respond the same as control animals after PHZ induced red blood cell lysis. Analysis of the hematocrit in adult Lyn+/up and Lyn+/+ mice (12-15 weeks) at 0, 3, and 5 days after PHZ treatment (n > 6, *P < .05). (C) Extramedullary early erythroid progenitor (BFU-E) dynamics after PHZ treatment is similar in Lyn+/up and control mice. Early erythroid colony assays (BFU-E) of spleens in Lyn+/+ and Lyn+/up adult mice (12-15 weeks) (n = 6, *P < .05). (D) Extramedullary late erythroid progenitor (CFU-E) dynamics after PHZ treatment is elevated in Lyn+/up compared with control mice. Late erythroid colony assays (CFU-E) of spleens in Lyn+/+ and Lyn+/up adult mice (12-15 weeks) (n = 6, *P < .05). (E) Elevated maturing erythroid cells in the spleen of Lyn+/up adult mice 3 days after PHZ treatment. Representative flow cytometric analysis of spleen cells from Lyn+/+ and Lyn+/up mice (12-15 weeks) at 3 days after PHZ treatment using anti-CD71 and anti-Ter119 and enumeration of the indicated erythroid subsets (R0, R1, R2, R3, R4; n > 6, *P < .05).49 (F) Elevated maturing erythroid cells in the spleen of Lyn+/up adult mice 5 days after PHZ treatment. Representative flow cytometric analysis of spleen cells from Lyn+/+ and Lyn+/up mice (12-15 weeks) at 5 days after PHZ treatment using anti-CD71 and anti-Ter119 and enumeration as in E (n > 6, *P < .05).
Altered response to chemically induced anemia in Lyn+/up adult mice. (A) Lyn+/up mice respond the same as control animals to PHZ treatment induced splenomegaly. Spleen wet weight of Lyn+/up and Lyn+/+ adult mice (12-15 weeks) at 0, 3, and 5 days after PHZ treatment (n > 6, *P < .05). (B) Lyn+/up hematocrits respond the same as control animals after PHZ induced red blood cell lysis. Analysis of the hematocrit in adult Lyn+/up and Lyn+/+ mice (12-15 weeks) at 0, 3, and 5 days after PHZ treatment (n > 6, *P < .05). (C) Extramedullary early erythroid progenitor (BFU-E) dynamics after PHZ treatment is similar in Lyn+/up and control mice. Early erythroid colony assays (BFU-E) of spleens in Lyn+/+ and Lyn+/up adult mice (12-15 weeks) (n = 6, *P < .05). (D) Extramedullary late erythroid progenitor (CFU-E) dynamics after PHZ treatment is elevated in Lyn+/up compared with control mice. Late erythroid colony assays (CFU-E) of spleens in Lyn+/+ and Lyn+/up adult mice (12-15 weeks) (n = 6, *P < .05). (E) Elevated maturing erythroid cells in the spleen of Lyn+/up adult mice 3 days after PHZ treatment. Representative flow cytometric analysis of spleen cells from Lyn+/+ and Lyn+/up mice (12-15 weeks) at 3 days after PHZ treatment using anti-CD71 and anti-Ter119 and enumeration of the indicated erythroid subsets (R0, R1, R2, R3, R4; n > 6, *P < .05).49 (F) Elevated maturing erythroid cells in the spleen of Lyn+/up adult mice 5 days after PHZ treatment. Representative flow cytometric analysis of spleen cells from Lyn+/+ and Lyn+/up mice (12-15 weeks) at 5 days after PHZ treatment using anti-CD71 and anti-Ter119 and enumeration as in E (n > 6, *P < .05).
Lyn+/up erythroid cell lines have greater viability but delayed differentiation in response to Epo
To determine the consequences of hyperactive Lyn on Epo-initiated signaling cascades, several independent cell lines were generated from control and Lyn+/up mice by immortalization of erythroid fetal liver cells with the J2 retrovirus.20 The morphology of the J2-Lyn+/up cell lines was similar to the lines generated from control mice (J2-WT; Figure 5A). In addition, flow cytometric analyses revealed that these cell lines had similar cell surface marker profiles (Figure 5B), as well as growth and clonogenicity (data not shown). Together, these results suggest that the cell lines from control and Lyn+/up mice were immortalized at similar stages of maturation.
Increased Epo-independent viability and delayed differentiation of erythroid Lyn+/up cells compared with Lyn+/+ cells. (A) Similar cell morphology of J2-WT and J2-Lyn+/up cell lines. Morphology of immortalized fetal liver erythroid J2-WT and J2-Lyn+/up cell lines stained with Wright-Giemsa (scale bar = 5 µm). (B) Equivalent cell surface marker expression in J2-WT and J2-Lyn+up cells. Flow cytometric profiles of J2-WT and J2-Lyn+/up cells stained for cell surface expression of Sca1, c-Kit, CD71, Ter119, CD11b, and CD44. (C) J2-Lyn+up cells (UP) have increased Epo-independent viability and delayed Epo-induced differentiation compared with J2-WT cells (WT). (i) Viable cell counts, (ii) viability (eosin dye exclusion), and (iii) differentiation (hemoglobin benzidine positive) analysis of J2-WT (WT) and J2-Lyn+/up (UP) cell lines in differentiation media (T3-depleted)21 in the presence (+E) or absence (−E) of Epo (5 U/mL). (D) Dose-response analysis of Epo on (i) viable cell counts, (ii) viability, and (iii) differentiation in differentiation media of J2-WT and J2-Lyn+/up cell lines. Cell culture conditions as for C, with the indicated concentrations of Epo at 48 (viable cells and viability) and 72 hours (differentiation).
Increased Epo-independent viability and delayed differentiation of erythroid Lyn+/up cells compared with Lyn+/+ cells. (A) Similar cell morphology of J2-WT and J2-Lyn+/up cell lines. Morphology of immortalized fetal liver erythroid J2-WT and J2-Lyn+/up cell lines stained with Wright-Giemsa (scale bar = 5 µm). (B) Equivalent cell surface marker expression in J2-WT and J2-Lyn+up cells. Flow cytometric profiles of J2-WT and J2-Lyn+/up cells stained for cell surface expression of Sca1, c-Kit, CD71, Ter119, CD11b, and CD44. (C) J2-Lyn+up cells (UP) have increased Epo-independent viability and delayed Epo-induced differentiation compared with J2-WT cells (WT). (i) Viable cell counts, (ii) viability (eosin dye exclusion), and (iii) differentiation (hemoglobin benzidine positive) analysis of J2-WT (WT) and J2-Lyn+/up (UP) cell lines in differentiation media (T3-depleted)21 in the presence (+E) or absence (−E) of Epo (5 U/mL). (D) Dose-response analysis of Epo on (i) viable cell counts, (ii) viability, and (iii) differentiation in differentiation media of J2-WT and J2-Lyn+/up cell lines. Cell culture conditions as for C, with the indicated concentrations of Epo at 48 (viable cells and viability) and 72 hours (differentiation).
J2-Lyn+/up cells exhibited enhanced viability compared with J2-WT cells in the absence of Epo and had a delayed differentiation response to Epo (Figure 5C). Dose-response analyses confirmed the enhanced viability of J2-Lyn+/up cells with low doses of Epo (Figure 5D), whereas differentiation was Epo dependent for both J2-WT and J2-Lyn+/up lines (Figure 5D).
Epo receptor signaling in Lyn+/up cell lines and primary Lynup/up erythroblasts: activation of JAK2 and Akt pathways and inhibition of GAB2
To examine the effects of hyperactive Lyn on Epo signaling, biochemical studies were performed (Figure 6A). Anti-phosphotyrosine immunoblots revealed that 2 proteins (pp39 and pp90) were heavily phosphorylated in J2-Lyn+/up cells before exposure to Epo, suggesting that they are direct Lyn target proteins. Interestingly, although the phosphorylation of both pp39 and pp90 increased following Epo stimulation in J2-WT cells, in J2-Lyn+/up cells, pp90 underwent a transient dephosphorylation, whereas the phosphorylation of pp39 remained stable. As expected, Lyn (pp53/56) phosphorylation and Epo receptor (pp68/70) phosphorylation increased in Epo-stimulated J2-WT cells, whereas in J2-Lyn+/up cells, Lyn underwent a transient dephosphorylation, and very little phospho-EpoR was detected. These data indicate that hyperactive Lyn can influence intracellular signaling in the absence of Epo, as well as the kinetics of protein phosphorylation following exposure to Epo.
Altered Epo receptor signaling in erythroid Lyn+/up cells compared with Lyn+/+ cells. (A) Altered phosphotyrosine dynamics during Epo induction and elevated EKLF, BclXL, CIS, SOCS1, and SOCS3 in J2-Lyn+/up cells. Time course of Epo-induced total phospho-tyrosine (pY) changes in J2-WT and J2-Lyn+/up cell lines. Prominent changes in phospho-proteins between the cell lines are indicated (arrows, and approximate kilodaltons). (B) Altered Lyn and proximal Epo receptor signaling dynamics during Epo induction in J2-Lyn+/up cells. Immunoblot analysis of J2-WT and J2-Lyn+/up cell lines for the signaling molecules indicated before and after 10 and 30 minutes of Epo stimulation. Total cell lysates were used for analysis except for pEpo receptor, where immunoprecipitates of the Epo receptor were blotted with anti-pY antibodies. (C) Altered downstream Epo receptor signaling dynamics during Epo induction in J2-Lyn+/up cells. Immunoblot analysis of total cell lysates of J2-WT and J2-Lyn+/up cell lines 0, 10, and 30 minutes after Epo stimulation for the signaling molecules indicated and GAB2 immunoprecipitates probed for total phosphotyrosine (pY) and GAB2. Immunoblot analysis was performed on 2 independent experiments producing equivalent results. (D) Increased levels of Epo receptor downstream targets in J2-Lyn+/up cells. Immunoblot analysis of total cell lysates of J2-WT and J2-Lyn+/up cell lines 0, 10, and 30 minutes after Epo stimulation for BclXL, SOCS1, SOCS3, and CIS.
Altered Epo receptor signaling in erythroid Lyn+/up cells compared with Lyn+/+ cells. (A) Altered phosphotyrosine dynamics during Epo induction and elevated EKLF, BclXL, CIS, SOCS1, and SOCS3 in J2-Lyn+/up cells. Time course of Epo-induced total phospho-tyrosine (pY) changes in J2-WT and J2-Lyn+/up cell lines. Prominent changes in phospho-proteins between the cell lines are indicated (arrows, and approximate kilodaltons). (B) Altered Lyn and proximal Epo receptor signaling dynamics during Epo induction in J2-Lyn+/up cells. Immunoblot analysis of J2-WT and J2-Lyn+/up cell lines for the signaling molecules indicated before and after 10 and 30 minutes of Epo stimulation. Total cell lysates were used for analysis except for pEpo receptor, where immunoprecipitates of the Epo receptor were blotted with anti-pY antibodies. (C) Altered downstream Epo receptor signaling dynamics during Epo induction in J2-Lyn+/up cells. Immunoblot analysis of total cell lysates of J2-WT and J2-Lyn+/up cell lines 0, 10, and 30 minutes after Epo stimulation for the signaling molecules indicated and GAB2 immunoprecipitates probed for total phosphotyrosine (pY) and GAB2. Immunoblot analysis was performed on 2 independent experiments producing equivalent results. (D) Increased levels of Epo receptor downstream targets in J2-Lyn+/up cells. Immunoblot analysis of total cell lysates of J2-WT and J2-Lyn+/up cell lines 0, 10, and 30 minutes after Epo stimulation for BclXL, SOCS1, SOCS3, and CIS.
Previously we showed that Lyn−/− erythroid cell lines have reduced GATA-1 and EKLF.9 Here we demonstrate that J2-Lyn+/up cells have the same level of GATA-1 expression as J2-WT cells, but elevated EKLF (Figure 6A).
We then explored these changes in greater detail, using antibodies directed against specific signaling molecules (Figure 6B). As anticipated, higher levels of activated Lyn (pSFK) were detected in J2-Lyn+/up cells in the absence of Epo stimulation. However, the levels of Lyn protein were noticeably lower in J2-Lyn+/up cells than J2-WT cells. This probably represents an intracellular response to ameliorate the impact of constitutively active Lyn and is also seen in bone marrow–derived macrophages and primary B cells purified from Lyn+/up mice.13,22 Interestingly, the kinetics of Lyn inactivation (pLyn-Y508) was also disrupted considerably in J2-Lyn+/up cells, which express 1 wild-type Lyn allele (Lyn-Y508) and 1 mutated Lyn allele (Lyn-F508; Figure 6B).
Typical Epo receptor phosphorylation occurred after Epo stimulation of J2-WT cells (Figure 6B). However, limited phosphorylation of the receptor was detected in J2-Lyn+/up cells, and the kinetics of phosphorylation was altered (Figure 6B). In addition, a significant increase in proteolytically processed Epo receptor (tEpo-R, ∼40 kDa) was detected in both unstimulated and Epo-stimulated J2-Lyn+/up cells, suggesting dramatically elevated turnover of the receptor.23
Because JAK2 is recognized as the primary kinase that phosphorylates the Epo receptor, the kinetics of JAK2 activation was analyzed. J2-WT cells displayed typical JAK2 phosphorylation in response to Epo stimulation, whereas J2-Lyn+/up cells displayed elevated JAK2 phosphorylation prior to Epo stimulation and then underwent a transient decrease after exposure to Epo (Figure 6B). Surprisingly, constitutively activated JAK2 in J2-Lyn+/up cells did not appear to increase basal Epo receptor phosphorylation; one explanation for this observation could be enhanced turnover of activated Epo receptors, as indicated by the elevated levels of truncated Epo-R. Alternatively, although JAK2 is phosphorylated in the absence of Epo in J2-Lyn+/up cells, the Epo-R still requires the presence of Epo to induce an appropriate reorientation that mediates receptor phosphorylation and subsequent downstream pathway activation.24 Further, the constitutive JAK2 phosphorylation was diminished in the presence of the JAK inhibitor, as well as the Lyn/SFK inhibitor PP2 (supplemental Figure 2A).
Both JAK2 and Lyn have been shown to phosphorylate STAT5.6-9,25,26 Interestingly, a marked increase in STAT5 phosphorylation occurred after exposure of J2-Lyn+/up cells to Epo (Figure 6B), which was markedly reduced by JAK2 and Lyn inhibition (supplemental Figure 2B). This observation indicates that hyperactive Lyn significantly alters the degree of STAT5 phosphorylation. Moreover, STAT5 phosphorylation is Epo dependent, but independent of constitutive JAK2 phosphorylation. Epo-R signaling can also stimulate STAT1 and STAT3 phosphorylation,27 and we show that J2-Lyn+/up cells have enhanced Epo-induced STAT3 phosphorylation, whereas STAT1 phosphorylation is reduced (Figure 6B).
The regulation of downstream pathways from the Epo-R is controlled by recruitment and phosphorylation of not only the receptor itself but also several adaptors/scaffolds and phosphatases. Unexpectedly, Epo-induced tyrosine phosphorylation of the adaptor GAB2 (at Y452 and total pY levels) was virtually absent in J2-Lyn+/up cells, whereas its serine phosphorylation (S159) was markedly elevated, and intriguingly, substantial serine phosphorylation was evident prior to Epo stimulation, which was further enhanced after Epo treatment (Figure 6C). This serine phosphorylation of GAB2 was sensitive to both JAK2 and Lyn inhibition (supplemental Figure 2B). GAB2 is known to be a Lyn substrate involved in the recruitment of the regulatory p85 subunit of PI3 kinase and the phosphatase Src homology region 2 domain-containing phosphatase 2 (SHP-2).28-30 Because Akt-mediated serine phosphorylation of GAB2 prevents its tyrosine phosphorylation, this may explain the lack of appreciable tyrosine phosphorylation of GAB2 in the J2-Lyn+/up cells.31 SHP-1 and SHP-2 are involved in modulation of Epo-R phosphorylation,32 Lyn activation,29,33 and regulation of downstream pathways.3,30 Interestingly, marginal Epo-induced phosphorylation of SHP-1 was observed in both J2-WT and J2-Lyn+/up cells, whereas robust SHP-2 phosphorylation occurred in both cell types, although this response was reduced considerably in Lyn+/up cells. This may be explained by reduced recruitment of SHP-2 to tyrosine phosphorylation sites on GAB2.
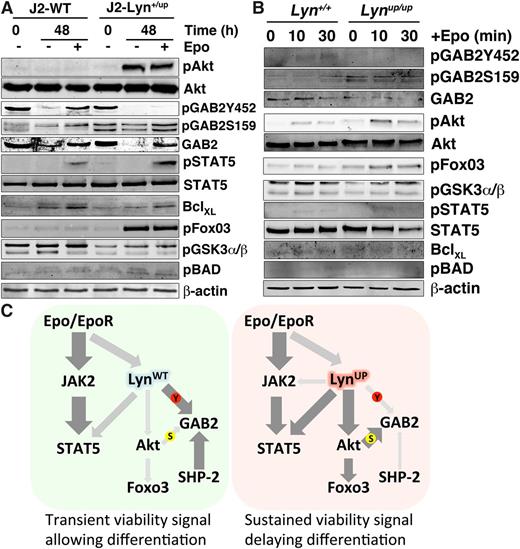
Other downstream signaling events were also monitored in J2-Lyn+/up cells. Markedly elevated phosphorylation of Akt and its downstream target FoxO3 were detected in J2-Lyn+/up cells before, and especially after, Epo induction (Figure 6C). The Epo-induced elevated pAkt in J2-Lyn+/up cells was dependent on both JAK2 and Lyn, because inhibition of either almost completely abrogated pAkt levels (supplemental Figure 2B). The increased Akt activity is unlikely to be via GAB2 recruitment of PI3K, because this is likely diminished in J2-Lyn+/up cells but could be direct or via intermediate molecules, as SFKs can directly phosphorylate and activate Akt,34 as well as the Akt activator PDK1.35 In contrast, the activation dynamics of Erk1/2 appeared similar in J2-WT and J2-Lyn+/up cells; although phosphorylation of p38MAPK was elevated in J2-Lyn+/up cells, pJNK levels were not altered (Figure 6C). The protein products of genes classically activated by the Epo receptor were also elevated in J2-Lyn+/up cells, ie, B-cell lymphoma-extra large (BclXL), cytokine-inducible SH2-containing protein (CIS), suppressor of cytokine signaling (SOCS)1, and SOCS3 (Figure 6D). Elevated levels of the antiapoptotic molecule BclXL may contribute to the Epo-independent viability36 of J2-Lyn+/up (Figure 5C-E), whereas increased CIS, SOCS1, and SOCS3 may represent an attempt to down-regulate intracellular signaling cascades.37 Collectively, these data demonstrate that constitutively active Lyn has a significant impact on Epo-independent and -dependent intracellular signaling. Because J2E cells are immortalized at the proerythroblast stage,20 these data suggest that Lyn plays a major role in transmitting survival signals via STAT5 and Akt in immature erythroid cells.
To investigate this further, signaling molecules were then examined over an extended time frame in high differentiation capacity media. In the presence or absence of Epo, Akt was highly active in J2-Lyn+/up cells after 48 hours of culture, whereas STAT5 phosphorylation was Epo dependent in both control and J2-Lyn+/up cell lines (Figure 7A). Pathways downstream of Akt were then investigated, revealing significantly enhanced phosphorylation of FoxO3 and reduced tyrosine but elevated inhibitory serine phosphorylation of GAB2 in J2-Lyn+/up cells. A modest increase in phosphorylated Bcl-2-associated death promoter (BAD) was seen, whereas phosphorylated GSK3α/β was decreased. These data suggest a viability pathway involving Akt-FoxO3 is markedly activated in Lyn+/up cells, thereby mediating elevated cell survival (Figure 5C-E).
Activation of Akt-FoxO3 in Lyn+/up erythroid cells during differentiation and in primary Lynup/up erythroblasts stimulated with Epo and model of altered signaling in Lyn+/up cells. (A) Epo-independent activation of Akt-FoxO3 in J2-Lyn+/up erythroid cells grown in differentiation media. Immunoblot analysis of total cell lysates of J2-WT and J2-Lyn+/up cell lines for the signaling molecules indicated, cultured in differentiation media (T3-depleted)21 with and without Epo at 0 and 48 hours. Immunoblot analysis was performed on 2 independent experiments producing equivalent results. (B) Altered Epo receptor signaling to GAB2 and Akt/FoxO3 in primary Lynup/up CD71+ splenic erythroblasts. Immunoblot analysis of total cell lysates of Lyn+/+ and Lynup/up CD71+ spleen cells 0, 10, and 30 minutes after Epo stimulation for the signaling molecules indicated. Immunoblot analysis was performed on 2 independent experiments producing equivalent results. (C) Model of altered signaling in (right) J2-Lyn+/up cells compared with (left) J2-LynWT cells. Thickness and darkness of arrows indicate relative intensity of signaling connections. S, serine phosphorylation; Y, tyrosine phosphorylation.
Activation of Akt-FoxO3 in Lyn+/up erythroid cells during differentiation and in primary Lynup/up erythroblasts stimulated with Epo and model of altered signaling in Lyn+/up cells. (A) Epo-independent activation of Akt-FoxO3 in J2-Lyn+/up erythroid cells grown in differentiation media. Immunoblot analysis of total cell lysates of J2-WT and J2-Lyn+/up cell lines for the signaling molecules indicated, cultured in differentiation media (T3-depleted)21 with and without Epo at 0 and 48 hours. Immunoblot analysis was performed on 2 independent experiments producing equivalent results. (B) Altered Epo receptor signaling to GAB2 and Akt/FoxO3 in primary Lynup/up CD71+ splenic erythroblasts. Immunoblot analysis of total cell lysates of Lyn+/+ and Lynup/up CD71+ spleen cells 0, 10, and 30 minutes after Epo stimulation for the signaling molecules indicated. Immunoblot analysis was performed on 2 independent experiments producing equivalent results. (C) Model of altered signaling in (right) J2-Lyn+/up cells compared with (left) J2-LynWT cells. Thickness and darkness of arrows indicate relative intensity of signaling connections. S, serine phosphorylation; Y, tyrosine phosphorylation.
To demonstrate that changes in signaling observed in cell lines reflected effects seen in vivo, we examined signaling in primary CD71+ erythroid cells isolated from the spleens of PHZ-treated mice. These studies revealed that the alterations to GAB2 serine and tyrosine phosphorylation and the Akt/FoxO3 pathways that we originally observed in the immortalized cells were recapitulated in these primary cells (Figure 7B).
Discussion
In previous studies, we established that Lyn is a critical signaling molecule involved in erythroid homeostasis.6,9,10 To assess this further and to unravel the signaling pathways in erythroid cells controlled by Lyn, we examined mice expressing constitutively active Lyn. We demonstrate that expression of gain-of-function Lyn leads to the development of anemia in adult mice and alters erythropoiesis during embryonic development. The presence of dysmorphic spherocyte-like cells and acanthocytes in the peripheral blood of Lynup/up mice suggests that hyperactive Lyn affects the membrane/cytoskeleton of red blood cells, indicating an intrinsic defect that results in hemolytic anemia. Lyn is the most active SFK in erythrocytes and serves to phosphorylate erythrocyte Band 3, a cytoskeletal-linked anion exchanger.38,39 Significantly, erythrocyte membrane changes in the erythroid condition chorea-acanthocytosis results from increased Lyn activity altering the phosphorylation status and organization of cytoskeletal proteins.40 Consequently, this animal model of elevated Lyn activity resembles a human red cell disorder and suggests that targeting Lyn may be a possible therapy. The presence of high numbers of enucleated erythrocytes in the fetal livers of Lynup/up mice may reflect an accelerated transition to definitive erythropoiesis in these mice due to premature maturation of primitive erythroid cells, which is known to be mediated by SFKs.41 Thus, hyperactive Lyn has a profound impact on the morphology of fetal and adult erythroid cells resulting in a hemolytic anemia.
Within adult Lynup/up mice, there is a compensatory expansion of bone marrow erythropoiesis, as well as a significant expansion of emergency erythropoiesis in the spleen, and Lyn+/up mice display a milder intermediate phenotype compared with Lynup/up animals, showing that expression of 1 gain-of-function Lyn allele is sufficient to induce significant alterations to the erythroid compartment. However, this elevated erythropoiesis is not sufficient to alleviate the appearance of anemia in these mice. Although this phenotype is similar to that of Lyn−/− mice,9,10 the underlying mechanism is likely to be different. A lack of Lyn elicits a stem/progenitor-intrinsic defect that reduces progenitor expansion and late-stage development, resulting in age-dependent anemia.8-10 However, for Lynup/up mice, the hyperactive Lyn elicits a red blood cell membrane defect producing acanthocytes and hemolysis; consequently, bone marrow and extramedullary erythroid progenitors are expanded in response to the hemolytic anemia. This indicates that the activity of Lyn needs to be carefully controlled to ensure normal erythropoiesis.9,10 As the mice age, anemia persists, and there is the suggestion of bone marrow exhaustion as BFU-E numbers decrease, whereas emergency erythropoiesis in the spleen increases still further compared with younger animals.
Taken together, these erythroid perturbations indicate that expression of hyperactive Lyn instigates a red blood cell defect that is only partially compensated by an expansion of erythropoiesis in the bone marrow and spleen and suggests that regulation of Lyn activity during erythropoiesis is critical for maintenance of erythroid homeostasis.
Interestingly, immortalized erythroid lines from Lyn+/up animals exhibited enhanced viability in the absence of Epo, albeit delayed differentiation in the presence of this cytokine. Our biochemical data demonstrate that constitutively elevated Lyn activity significantly alters Epo receptor phosphorylation, the JAK/STAT pathway, the signaling adaptor GAB2, and the Akt/FoxO3 cascade, leading to expression of the antiapoptotic protein BclXL. Importantly, alterations to GAB2 and Akt/FoxO3 were recapitulated in primary Lynup/up erythroblasts. Interestingly, the activated Akt in Lyn+/up cells fed into a FoxO3 pathway but produced little or no GSK3α/β or BAD activation, two other classical Akt substrates. This separation of Akt pathways activated by Lyn may be due to a subcellular localization divergence, as FoxO3 is a nuclear protein, whereas GSK3α/β and BAD are located in the cytoplasm. Potentially this may be mediated by differential GAB2 phosphorylation, which is known to be 1 link between the Epo-R and PI3 kinase/Akt, because in Lyn+/up cells, there is a near absence of Epo-induced phosphotyrosine-GAB2, whereas phosphoserine-GAB2 is substantially elevated prior to Epo stimulation.3,10,30,42 Interestingly, although we previously showed that SHP-1/2 are hyperphosphorylated in macrophages from Lynup/up mice, here in erythroid cells, neither showed an elevated phosphorylation status prior to or after Epo stimulation in J2-Lyn+/up cells.13 The lack of GAB2 phosphorylation seems counterintuitive, as GAB2 is a substrate of Lyn.28 GAB2 can also mediate differential PI3 kinase/Akt signaling toward proliferation/viability or differentiation outcomes.28 Significantly, elevation of Akt activity is used in a negative feedback loop to modulate GAB2 tyrosine phosphorylation and its interactions with other proteins.31 The elevated Akt activity in resting J2-Lyn+/up cells and primary Lynup/up erythroblasts, as well as their very high Epo-induced Akt activation, may promote the serine phosphorylation of GAB2, which then prevents Lyn-mediated GAB2 tyrosine phosphorylation, keeping it in its negative feedback state and inhibiting its subsequent regulation of downstream pathways. This may also result in reduced recruitment of SHP-1/2 to the receptor complex, potentially explaining the lowered SHP-2 phosphorylation in J2-Lyn+/up cells and in part the elevated Epo-induced STAT5 and STAT3 phosphorylation in J2-Lyn+/up cells. It is conceivable that the increased viability initiated by the Akt-FoxO3 pathway occurs by regulating expression of apoptotic genes, eg, Bim and PUMA.43-45 A lack of Lyn reduced Epo-mediated STAT5 activation,9 which is consistent with the elevated STAT5 phosphorylation seen in J2-Lyn+/up cells. However, elevated Akt activity was observed when Lyn was ablated.8 It may be that Lyn mediates both positive and negative regulation of Akt, consistent with its known roles in both activating and inhibiting signaling pathways.46-48 We propose that because of this altered intracellular signaling caused by a gain-of-function Lyn, through hyperactivation of Akt and reduced tyrosine phosphorylation of GAB2, the balance between viability, expansion, and differentiation of Epo-responsive cells in vivo is severely perturbed (Figure 7C). As a consequence, erythroid progenitor and precursor levels in mice harboring either 1 or 2 Lynup alleles are disrupted, and the animals become increasingly anemic.
We showed previously that loss of Lyn severely impacts erythropoiesis.9,10 Here, we demonstrated that constitutively active Lyn affects signaling in erythroid cells, influences cell viability, and leads to major compensatory changes in the erythroid compartment. Together, these results illustrate that regulation of Lyn activity and gene dosage are crucial for normal erythropoiesis. Insufficient Lyn or inappropriately activated Lyn are similarly deleterious to the erythroid compartment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Adley Handoko, Alison Louw, and Irma Larma for technical support.
This work was supported by grants from the National Health and Medical Research Council (513714 and 634352), the Medical Research Foundation of Royal Perth Hospital, and the Cancer Council of Western Australia. E.I. is supported by a senior fellowship from the Cancer Council of Western Australia. M.L.H. is supported by a research fellowship from the National Health and Medical Research Council, Australia.
Authorship
Contribution: N.S.S.-A. and M.J.M. designed, supported, and performed experiments, analyzed data, and contributed to writing the manuscript; A.M., C.Q., J.S., L.S., and N.K. performed experiments; W.E. analyzed results and contributed to writing the manuscript; S.P.K. and M.L.H. supported the research, designed experiments, analyzed results, and contributed to writing the manuscript; E.I. designed and supported the research, designed and undertook experiments, analyzed data, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Evan Ingley, Cell Signalling Group, WAIMR, Level 6 MRF Building, Rear 50 Murray St, Perth, WA 6000, Australia; e-mail: evan.ingley@uwa.edu.au.

![Figure 1. Alterations to erythroid tissues in Lynup/up mice. (A) Peripheral blood from adult Lynup/up mice contains acanthocytes and spherocyte-like erythrocytes. Blood smears from adult Lyn+/+ and Lynup/up mice stained with Wright-Giemsa (black arrowhead = acanthocyte, white arrowhead = spherocyte-like, scale bar = 10 µm). (B) The bone marrow of adult Lynup/up mice displays altered ratios of erythroid and granulocytic cells compared with Lyn+/+ animals. Cytopreps of single cell suspensions of bone marrow from adult Lyn+/+ and Lynup/up mice were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = erythroblasts, scale bar = 20 µm). (C) Lynup/up spleens contain normal and abnormal erythroid/blast cells. Cytopreps of single cell suspensions of spleen from adult Lyn+/+ and Lynup/up mice were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = erythroblasts, black arrowhead = abnormal erythroblasts, yellow arrowhead = blasts, scale bar = 20 µm). (D) Lynup/up fetal livers (E12.5) contain significant numbers of definitive (enucleated) erythrocytes compared with Lyn+/+ animals. Cytopreps of single cell suspensions of fetal liver (E12.5) of Lyn+/+ and Lynup/up embryos were stained for hemoglobin with neutral benzidine and cell morphology using Wright-Giemsa (white arrowhead = definitive [enucleated] erythrocytes, scale bar = 20 µm).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2012-10-463158/4/m_262f1.jpeg?Expires=1765900902&Signature=OsU48EXxqny3ZS8ansLRMs53-88ZRqORMhwssnXhnk3KKuPe39N6Uyi~6GLB30TFnVLefNIgO~QpQ1rPySHppm6NtYrJd7c9-7mCogutHzuM9hJAJAr5jufUGoRZPxfYwPeefq3pEstQJbw7VsAkDMwxR1iZydpqVVFtmxpjMOZhxtdqZMJxsHzPBVFJ5NNEWTw24S750YkEUZ-aq98wuwFOCOMhnKstFN~yOyBfRAlmY0V3wOmCLiGRl2s1tUb1ESqUcZhjdZxAPn77ohYn2SSeCop1DPCz-VCtoXMQomZgY93SJ~FlXyCG7GDyDIN7-TBdzH8CZI5yQLwekWo89Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal