Key Points
Infiltrating FLT3-ITD neutrophils identified in skin confirms terminal differentiation of FLT3-ITD blasts after FLT3 inhibitor therapy.
Neutrophilic dermatosis after FLT3 inhibition may be a manifestation of a differentiation syndrome associated with this treatment.
Abstract
The FLT3-ITD mutation is associated with poor outcomes in acute myeloid leukemia. Multiple FMS-like tyrosine kinase 3 (FLT3)-inhibitors have been studied in clinical trials. Recently, potent FLT3 inhibition was shown to induce terminal differentiation of FLT3-mutant myeloblasts. In 3 patients who developed characteristic skin nodules on initiation of FLT3-inhibition, we conducted dermatopathologic evaluation of skin samples, as well as FLT3 and NPM1 mutational analysis and fluorescence in situ hybridization. All 3 patients demonstrated characteristically deep dermal and subcutaneous neutrophilic infiltrates without evidence of myeloblasts. Discovery of FLT3-ITD and NPM1 mutations in 2 of the samples, as well as the presence of FLT3-ITD and deletion of 7q in the other, confirmed the ancestry of the differentiated neutrophils as that of the original FLT3-mutant myeloblasts. FLT3 inhibition can lead to clinically distinct dermatoses, which suggests the effect of FLT3 inhibition on myeloid differentiation and a manifestation of a broader “syndrome” associated with this therapy.
Introduction
The FMS-like tyrosine kinase 3 (FLT3) gene is mutated in approximately 30% of patients with acute myeloid leukemia (AML), with the majority being internal tandem duplication (ITD) mutations,1 an alteration associated with higher relapse rates and worse outcomes.2-4 The FLT3-ITD protein product is a ligand-independent, constitutively active receptor tyrosine kinase causing activation of multiple signaling cascades, suppression of apoptosis, and proliferation of myeloblasts.5,6 Several inhibitors of the FLT3-ITD protein are under clinical investigation.7-10
FLT3 inhibition causes direct cytotoxicity to myeloblasts.11,12 However, intriguingly, terminal differentiation of FLT3-ITD myeloblasts was recently reported in patients treated with quizartinib, a potent FLT3 inhibitor.13 We report the incidence of neutrophilic dermatoses in 3 FLT3-ITD patients treated with FLT3 inhibitors and demonstrate that these infiltrating neutrophils retain FLT3-ITD expression, confirming the process of terminal differentiation induced by this therapy.
Study design
Skin biopsy samples from 3 patients seen at the Massachusetts General Hospital in Boston and Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, Maryland, were evaluated. All patients were diagnosed with FLT3-ITD AML, were treated with FLT3 inhibitors, and subsequently developed skin nodules. Samples were obtained through protocols approved by each institution’s institutional review board. The study was conducted in accordance with the Declaration of Helsinki.
FLT3-ITD mutation status was assessed using previously reported primers.14 NPM1 exon 12 insertion mutation status was assessed using custom-designed primers (forward 5′-AGTTAACTCTCTGGTGGTAGAATG-3′ [labeled with NED] and reverse 5′-GGACAGCCAGATATCAACTGTTAC-3′ [labeled with HEX]). Primer sets were combined to perform multiplex polymerase chain reaction (PCR; 94°C 2 minutes; 36 cycles × 94°C 15 seconds; 60°C 30 seconds; 72°C 30 seconds) for samples, with subsequent analysis by Applied Biosystems Genetic Analyzer capillary electrophoresis.
EGFR and MET gene copy number was evaluated by fluorescence in situ hybridization (FISH) on formalin-fixed paraffin-embedded tissue sections, as described previously.15
Results and discussion
Case histories are summarized here and in Table 1.
Clinical characteristics of 3 patients with FLT3-ITD mutations, treated with FLT3 inhibitors, who subsequently developed tender skin nodular consisting of FLT3-mutant infiltrating neutrophilic infiltrates
| Patient . | Age/sex . | WBC (/µL) at presentation . | WBC (/µL) (neutrophil, blast %) at time of skin lesion . | Karyotype . | FLT3 status . | NPM1 status . | FLT3 Inhibitor . | Time to onset of skin lesion, wk . |
|---|---|---|---|---|---|---|---|---|
| 1 | 29/female | 30 000 | 550 (4%, 0%) | Normal | ITD | Mutant | Quizartinib | 8 |
| 2 | 60/female | 40 000 | 570 (76%, 0%) | t (10;17), del (15) | ITD | Mutant | Quizartinib | 6 |
| 3 | 56/male | 6600 | 1100 (56%, 8%) | del (7q) | ITD | WT | Sorafenib | 2 |
| Patient . | Age/sex . | WBC (/µL) at presentation . | WBC (/µL) (neutrophil, blast %) at time of skin lesion . | Karyotype . | FLT3 status . | NPM1 status . | FLT3 Inhibitor . | Time to onset of skin lesion, wk . |
|---|---|---|---|---|---|---|---|---|
| 1 | 29/female | 30 000 | 550 (4%, 0%) | Normal | ITD | Mutant | Quizartinib | 8 |
| 2 | 60/female | 40 000 | 570 (76%, 0%) | t (10;17), del (15) | ITD | Mutant | Quizartinib | 6 |
| 3 | 56/male | 6600 | 1100 (56%, 8%) | del (7q) | ITD | WT | Sorafenib | 2 |
WT, wild-type.
Case 1
Patient 1 was a 29-year-old woman presenting with a white blood cell count (WBC) of 30 000/μL and diagnosed with cytogenetically normal AML, with FLT3-ITD and nucleophosmin (NPM1) mutations. After induction chemotherapy, she achieved complete remission (CR). She received a single cycle of high-dose cytarabine and underwent myeloablative haplo-identical stem cell transplantation. She experienced relapse 4 months later, and quizartinib was initiated. Of note, before initiation of quizartinib, the FLT3-ITD-to-wild-type ratio was 0.2 (ITD allelic burden of 20%). Two months later, she developed tender, erythematous nodules on her lower extremities, which resolved after therapy with oral dexamethasone. Quizartinib was ultimately discontinued because of worsening performance status, and she died soon thereafter.
Skin biopsy revealed subcutaneous, lobular mixed inflammatory infiltrates, including numerous neutrophils, consistent with lobular panniculitis. The infiltrates lacked morphologically identifiable blasts and lacked CD34 or CD117 expression (markers expressed in her leukemic blasts) by immunohistochemistry. Sizing analysis by PCR detected both FLT3-ITD and NPM1 insertional mutations in the skin sample.
Case 2
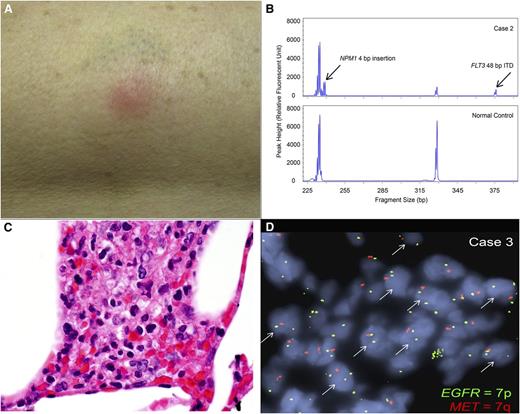
Patient 2 was a 60-year-old woman presenting with a WBC of 40 000/µL. She was diagnosed with FLT3-ITD and NPM1-mutant AML, characterized by translocation t(10;17) (q24;p13) and partial deletion of chromosome 15 (q15q24). She received induction chemotherapy and achieved CR. After 2 cycles of decitabine, she relapsed and received mitoxantrone, etoposide, and cytarabine reinduction. She achieved remission with incomplete peripheral hematopoietic recovery. Disease relapse followed within weeks, and quizartinib was started. Before initiation of quizartinib, the FLT3-ITD allelic burden was 95%. Six weeks later, pulmonary nodules were noted on imaging, as was a tender, erythematous skin nodule on her right thigh (Figure 1A), which was biopsied. Treatment with oral dexamethasone led to resolution of the skin nodule within days. She subsequently underwent allogeneic transplant but died in the peritransplant period from complications of sepsis.
Clinical and pathological manifestations of neutrophilic dermatoses. (A) Photograph of the erythematous, tender nodule on the outer right thigh of patient 2, arising 6 weeks after initiation of the FLT3 inhibitor quizartinib. (B) Fragment size analysis for FLT3 ITD and NPM1 insertion mutations. A representative analysis for FLT3 ITD and NPM1 insertion mutations using a PCR fragment size assay targeting FLT3 exon 15 and NPM1 exon 12 in genomic DNA extracted from the skin biopsy sample from patient 2. Arrows indicate the respective insertion mutations found in the patient but not in the normal control. (C) Histology of skin biopsy from patient 3. Clusters of neutrophils are present within subcutaneous fat, admixed with histiocytes. No blasts are identified (original magnification 1000×, images taken on Olympus BX41 microscope). (D) EGFR (7p) and MET (7q) FISH. Copy number analysis by FISH was performed on a skin biopsy formalin-fixed paraffin-embedded tissue section from patient 3, using probes specific for EGFR (green), which corresponds to chromosome 7p, and MET (red), which corresponds to chromosome 7q. The arrows indicate nuclei showing a single copy loss of MET or 7q, identified within the subcutaneous neutrophilic infiltrates.
Clinical and pathological manifestations of neutrophilic dermatoses. (A) Photograph of the erythematous, tender nodule on the outer right thigh of patient 2, arising 6 weeks after initiation of the FLT3 inhibitor quizartinib. (B) Fragment size analysis for FLT3 ITD and NPM1 insertion mutations. A representative analysis for FLT3 ITD and NPM1 insertion mutations using a PCR fragment size assay targeting FLT3 exon 15 and NPM1 exon 12 in genomic DNA extracted from the skin biopsy sample from patient 2. Arrows indicate the respective insertion mutations found in the patient but not in the normal control. (C) Histology of skin biopsy from patient 3. Clusters of neutrophils are present within subcutaneous fat, admixed with histiocytes. No blasts are identified (original magnification 1000×, images taken on Olympus BX41 microscope). (D) EGFR (7p) and MET (7q) FISH. Copy number analysis by FISH was performed on a skin biopsy formalin-fixed paraffin-embedded tissue section from patient 3, using probes specific for EGFR (green), which corresponds to chromosome 7p, and MET (red), which corresponds to chromosome 7q. The arrows indicate nuclei showing a single copy loss of MET or 7q, identified within the subcutaneous neutrophilic infiltrates.
Dermatopathologic evaluation revealed deep dermal and subcutaneous inflammatory infiltrates consisting of abundant neutrophils, but no blasts. Sizing analysis by PCR detected both FLT3-ITD and NPM1 insertional mutations (Figure 1B) in the skin sample.
Case 3
Patient 3 was a 56-year-old man with history of osteosarcoma, for which he underwent chemotherapy and resection. Two years later, he developed therapy-related AML, with deletion of 7q and no FLT3 or NPM1 mutations. He was treated with induction chemotherapy, achieving CR, followed by a myeloablative matched related donor stem cell transplantation. Four months later, he suffered relapse of his disease, which was refractory to mitoxantrone, etoposide, and cytarabine and then to decitabine. A bone marrow biopsy 11 months after diagnosis revealed persistent AML, but now with a FLT3-ITD mutation, with ITD allelic burden being 15%. The patient was started on lenalidomide and sorafenib. Two weeks later, he developed tender subcutaneous nodules on his left thigh, which were biopsied. Imaging revealed concurrent pulmonary nodules. The patient ultimately succumbed to progressive disease.
Dermatopathologic evaluation revealed dermal and subcutaneous lobular neutrophilic infiltration (Figure 1C) with no CD34-positive or CD117-positive blasts, consistent with lobular neutrophilic dermatosis and panniculitis. Flow cytometry on this specimen revealed no evidence of myeloblasts. PCR sizing analysis on the skin sample detected a FLT3-ITD mutation. FISH copy analysis for EGFR (located on 7p11.2) and MET (located on 7q21.11) revealed aggregates of cells within the neutrophilic infiltrates, with each nucleus showing 2 red EGFR signals and 1 green MET signal located on 7p and 7q, respectively, confirming clonal derivation from the original leukemic myeloblasts (Figure 1D).
Discussion
Potent FLT3 inhibition appears to cause terminal differentiation of arrested myeloid precursors in AML.13 The effect of FLT3-ITD mutation on differentiation is previously described, and release of differentiation block appears to be a feature of FLT3 inhibition.16,17 Differentiation and cell cycling are coordinated phenomena, and the transcription factor C/EBPα has been identified as an important mediator.17 Dysfunction of C/EBPα activity is well-described in AML, and FLT3-ITD mutations inhibit C/EBPα.17-19 FLT3 inhibition leads to cytotoxicity within days, with clearance of peripheral blasts,13,20,21 but its effect on terminal differentiation usually follows during a period of weeks.13 A similar time frame was noted in our patients, with characteristic skin findings occurring weeks after therapy initiation.
We here describe a dermatologic manifestation of a “differentiation syndrome” that appears to follow initiation of FLT3 inhibition. These patients all developed painful subcutaneous nodules within weeks of starting therapy, with pathological evaluation revealing deep neutrophilic infiltration and panniculitis. Skin biopsy samples contained no myeloblasts but did express FLT3-ITD, as well as other cytogenetic or molecular aberrations that had characterized the patients’ leukemic clone, suggesting that the infiltrating neutrophils were terminally differentiated from FLT3-mutant precursors. In addition, 2 of these patients developed concurrent pulmonary nodules without clear evidence of infection, suggesting this too as a manifestation of differentiation.
Intriguingly, in 2 patients, steroid therapy led to resolution of skin nodules. To date, in 22 patients treated with quizartinib at Johns Hopkins, 3 have developed biopsy-confirmed neutrophilic dermatoses, for an occurrence rate of 13.6%. At Massachusetts General Hospital, in 10 FLT3-ITD patients treated with single-agent sorafenib since 2007, a single patient developed confirmed neutrophilic dermatosis, for a rate of 10%.
On the basis of these limited data, we therefore estimate a likelihood of occurrence for this phenomenon in patients with relapsed/refractory AML treated with a FLT3 inhibitor of approximately 10% during the treatment period. Our patients were treated with single-agent quizartinib and sorafenib, with the subsequent development of characteristic skin nodules, suggesting that the effect is class-specific and a feature of potent FLT3 inhibition. We chose to include our experience with single-agent FLT3 inhibitors and not their combination with cytotoxic chemotherapy, such as that of the FLT3 inhibitor midostaurin and induction chemotherapy, for which we have not observed similar dermatologic phenomena. This is likely because concurrent cytoreduction with traditional chemotherapy suppresses differentiation and its clinical manifestations.
Our patients did have some unique clinical features, in that patients 2 and 3 displayed karyotypic abnormalities, and in the case of patient 3, FLT3-ITD was only detected at relapse. Although the majority of patients with FLT3-ITD AML have a normal karyotype and exhibit the mutation on presentation, studies have demonstrated that FLT3-ITD can be acquired on relapse and can accompany a range of karyotypic abnormalities.3,22,23 In addition, in our patients, the extent of FLT3-mutant allelic burden did not appear to correspond to the development of neutrophilic dermatosis, although it is difficult to draw conclusions from this limited number of cases.
This syndrome appears to share features with well-described phenomena associated with use of all-trans retinoic acid in acute promyelocytic leukemia, characterized by neutrophilic differentiation, with possible pulmonary and skin manifestations.24 All-trans retinoic acid-differentiated cells are known to overexpress C-X-C chemokine receptor 4 (CXCR4), which interacts with the stromal factor, SDF-1, residing in affected tissues.25 CXCR4 expression is elevated in FLT3-ITD AML,26,27 potentially priming these cells on differentiation for CXCR4-mediated migration and SDF-1 interaction in tissues.
In light of these and similar findings,13 we suggest that remissions resulting from FLT3 inhibition, as recently reported,7,21 may differ in quality from those achieved after conventional chemotherapy. The latter are “kinetic” remissions resulting from repopulation, by normal precursors, of a cytoreduced marrow milieu, whereas remissions resulting from FLT3-inhibitors may be driven by terminal differentiation and gradual replacement of FLT3-ITD precursors. This distinction may have broader clinical implications, and responses to FLT3-targeted therapies require further study to determine their clinical trajectory compared with more traditional states of remission.
In summary, FLT3 inhibition can lead to clinically and pathologically distinct dermatoses consisting of deeply infiltrative, terminally differentiated FLT3-mutant neutrophils. As use of FLT3 inhibitors expands, physicians will increasingly detect these manifestations, which may be part of a broader differentiation syndrome associated with this therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.T.F. developed the study design, performed data collection, and wrote the manuscript; L.L. performed molecular and FISH assays on tissue; R.P.H. performed histopathological evaluation, provided images, and helped edit the manuscript; H.S. helped with sample collection and editing of manuscript; and M.L. and Y.-B.C. helped with study design and development and edited the manuscript.
Conflict-of-interest disclosure: M.L. is on the clinical advisory board for Ambit Biosciences. Y.C. receives grant support from Bayer/Onyx to conduct clinical trials. The remaining authors declare no competing financial interests.
Correspondence: Amir T. Fathi, Massachusetts General Hospital, Harvard Medical School, Zero Emerson Place, Suite 118, Boston, MA 02114; e-mail: afathi@partners.org.
References
Author notes
A.T.F., M.L., and Y.-B.C. contributed equally to this study.