Key Points
WT1 mutations predict poor prognosis in AML; Wt1 mutant embryonic stem cells show reduced hematopoiesis, elevated apoptosis, and abnormal Vegf-a isoforms.
Wt1 regulates Vegf-a splicing; exogenous Vegf-a restores hematopoiesis in Wt1 mutant ES cells, and this pathway may have therapeutic potential.
Abstract
Mutations in the Wilms tumor suppressor 1 (WT1) gene are as frequent in acute myeloid leukemia (AML) as in nephroblastma and predict poor prognosis. However, the role of WT1 in hematopoiesis remains unclear. We show that Wt1-deficient mouse embryonic stem cells exhibit reduced hematopoietic potential caused by vascular endothelial growth factor A (Vegf-a)–dependent apoptosis of hematopoietic progenitor cells associated with overproduction of the Vegf-a120 isoform. We demonstrate that Wt1 promotes exon inclusion using a Vegf-a minigene-based splicing assay. These data identify a critical role for Wt1 in hematopoiesis and Vegf-a as a cellular RNA whose splicing is potentially regulated by Wt1. The correction of Wt1 deficiency by treatment with exogenous Vegf-a protein indicates that the Wt1/Vegf-a axis is a molecular pathway that could be exploited for the management/treatment of poor prognosis AMLs.
Introduction
Following the identification of the Wilms tumor suppressor 1 (WT1) gene,1 WT1 mutations have been found in ∼10% of acute myeloid leukemia (AML) patients, correlating with poor treatment outcome.2-4 Wt1 knockout mice display multiple developmental defects, including complete kidney agenesis, and die in utero, typically between embryonic days 12.5 and 13.5.5 Transplantation of hematopoietic cells from the fetal liver and aorta-gonads-mesonephros region indicates that murine hematopoietic progenitor cells lacking Wt1 can engraft irradiated recipients.6,7 However, Wt1-deficient fetal liver cells show impaired differentiation potential when assessed by in vitro colony assay.6 In contrast, embryonic stem (ES) cells lacking Wt1 activity display a mild delay in hematopoietic differentiation only.8 In order to reconcile these conflicting reports and clarify the role of Wt1, we analyzed the hematopoietic potential of ES cells lacking Wt19 in vivo and in vitro.
Study design
ES cells were maintained in the presence of leukemia inhibitory factor on gelatin-coated plates, differentiated in hanging drops without leukemia inhibitory factor (1000 cells per drop) for 24 hours prior to liquid suspension culture or 2-step hematopoietic colony assays (Stem Cell Technologies).
Vascular endothelial growth factor A (Vegf-a)164 (Peprotech) was added on day 3 of differentiation (final concentration 10 ng/mL).
Flow cytometry (fluorescence-activated cell sorter [FACS]) analysis was conducted with directly conjugated antibodies (BD Biosciences) on a FACSCalibur Flow Cytometer (BD Biosciences) with BD Cell Quest Pro software. Apoptosis was measured using the Annexin-V-APC Apoptosis Detection Kit (BD Biosciences).
Mice were housed under pathogen-free conditions, and all procedures were carried out under personal and project licenses issued by the Home Office, United Kingdom, and were approved by the Local Ethical Committee.
Results and discussion
Reduced hematopoietic development was evident after 6 days of differentiation in Wt1-null embryoid bodies (EBs) by flow cytometry (FACS) (Figure 1A), affecting the first emerging cells of the erythroid (Ter-119+ve) lineage as well as multipotent progenitors (CD41+ve c-Kit+ve). After 8 days of differentiation, a marked reduction in the percentage of mature myeloid (Mac-1+ve) cells was observed (Figure 1A). To functionally assess hematopoietic progenitors, dissociated day 8 EBs were seeded into methyl cellulose colony assays (Stem Cell Technologies). Consistent with EB FACS, Wt1-deficient EBs showed a general reduction (∼75%) in all classes of myeloid/erythroid hematopoietic progenitor cells compared with wild type (Figure 1B). Despite a dramatic reduction in the number of hematopoietic progenitors, Wt1-null cells were capable of giving rise to erythroid blast forming unit (BFU-E), myeloid colony forming unit (CFU-GM), and multipotent (CFU-mix) colonies morphologically indistinguishable from wild type (data not shown). Given that mice lacking Wt1 show complete kidney agenesis caused by apoptosis of the metanephric mesenchyme,5 we investigated whether apoptosis was the underlying cause of diminished hematopoiesis in Wt1-null EBs by staining the first Ter119+ve hematopoietic cells to emerge from EBs with annexin V (Figure 1C). More than 50% of Ter-119+ve progenitors in Wt1-null EBs were apoptotic compared with ∼25% in wild type. In contrast, there was no reduction in annexin-V staining of total, unfractionated EB cells, indicating that this was not simply a general increase in apoptosis in EBs lacking Wt1. Multipotency was confirmed by injecting green fluorescent protein (GFP)-labeled ES cells into wild-type blastocysts: Wt1-null ES cells contributed widely to chimeric tissues (visually estimated at up to 10%), with the exception of the kidney (supplemental Figure 1). Thus, Wt1-null ES cells retained multipotency, whereas exclusion of Wt1-null cells from the kidney indicates a cell autonomous requirement for Wt1 during nephrogenesis. Furthermore, FACS analysis revealed that Wt1-null cells have the capacity to contribute to fetal liver hematopoiesis (compare GFP+ve gate [derived from Wt1-null cells] with GFP–ve gate [endogenous, wild-type cells], Figure 1D and supplemental Figure 2), indicating that any requirement for Wt1 during hematopoietic differentiation is not cell autonomous.
Hematopoiesis is significantly perturbed in Wt1-deficient EBs. (A) Representative FACS plots of disaggregated wild-type and Wt1-nullk1 EBs stained at (i) day 6 of differentiation for CD41/c-Kit (n = 7; P < .0005), (ii) day 6 for Ter119 (n = 5; P < .01), and (iii) day 8 for Mac-1 (n = 5; P < .0005), showing fewer hematopoietic progenitor cells, mature erythroid cells, and myeloid cells in Wt1-null EBs. The numerical data are the mean of independent replicates, tested for significance using Student t test; 2-tailed distribution assuming unequal variance. P values represent both Wt1-null clones vs wild type. (B) Hematopoietic colony assay (Stem Cell Technologies) of disaggregated day 8 EBs showing numbers of erythroid (BFU-E), myeloid (CFU-GM), and multipotent (CFU-mix) progenitor cells per 3 × 105 EB cells. Both Wt1-null clones individually show a significant reduction in colony-forming potential (P < .005), including all individual colony types (P < .05), compared with wild type. The data are the mean of independent replicates, each conducted in triplicate. Bars display standard deviation; significance tested using analysis of variance (n [wild type vs K1] = 4; n [wild type vs K46] = 3). (C) Hematopoietic progenitor cells lacking Wt1 show elevated levels of apoptosis compared with wild type. Representative FACS plots for (i) annexin-V staining of cells gated for Ter119+ve expression showing increased levels of apoptosis in Wt1-null EBs compared with wild type (n = 5; P < .02) and (ii) annexin-V staining of unfractionated, disaggregated wild-type and Wt1-null EBs showing that the increased apoptosis of hematopoietic progenitors is not a general feature of all EB cells lacking Wt1 (n = 5; P > .05). The numerical data are the mean of independent replicates, tested for significance using Student t test; 2-tailed distribution assuming unequal variance. P values represent both Wt1-null clones vs wild type. (D) FACS analysis of e15.5 fetal livers from chimeric embryos showing capacity of donor Wt1-null ES cells (GFP+ve) to generate diverse hematopoietic cell types within chimeric fetal livers in similar proportions to the endogenous wild-type host (GFP–ve): (i) Mac-1 (myeloid lineage), (ii) c-Kit (progenitor cells), (iii) Ter-119 (erythroid lineage), and (iv) B220 (lymphoid lineage). Data from the chimera with the highest contribution of GFP+ve Wt1-null ES cells are shown.
Hematopoiesis is significantly perturbed in Wt1-deficient EBs. (A) Representative FACS plots of disaggregated wild-type and Wt1-nullk1 EBs stained at (i) day 6 of differentiation for CD41/c-Kit (n = 7; P < .0005), (ii) day 6 for Ter119 (n = 5; P < .01), and (iii) day 8 for Mac-1 (n = 5; P < .0005), showing fewer hematopoietic progenitor cells, mature erythroid cells, and myeloid cells in Wt1-null EBs. The numerical data are the mean of independent replicates, tested for significance using Student t test; 2-tailed distribution assuming unequal variance. P values represent both Wt1-null clones vs wild type. (B) Hematopoietic colony assay (Stem Cell Technologies) of disaggregated day 8 EBs showing numbers of erythroid (BFU-E), myeloid (CFU-GM), and multipotent (CFU-mix) progenitor cells per 3 × 105 EB cells. Both Wt1-null clones individually show a significant reduction in colony-forming potential (P < .005), including all individual colony types (P < .05), compared with wild type. The data are the mean of independent replicates, each conducted in triplicate. Bars display standard deviation; significance tested using analysis of variance (n [wild type vs K1] = 4; n [wild type vs K46] = 3). (C) Hematopoietic progenitor cells lacking Wt1 show elevated levels of apoptosis compared with wild type. Representative FACS plots for (i) annexin-V staining of cells gated for Ter119+ve expression showing increased levels of apoptosis in Wt1-null EBs compared with wild type (n = 5; P < .02) and (ii) annexin-V staining of unfractionated, disaggregated wild-type and Wt1-null EBs showing that the increased apoptosis of hematopoietic progenitors is not a general feature of all EB cells lacking Wt1 (n = 5; P > .05). The numerical data are the mean of independent replicates, tested for significance using Student t test; 2-tailed distribution assuming unequal variance. P values represent both Wt1-null clones vs wild type. (D) FACS analysis of e15.5 fetal livers from chimeric embryos showing capacity of donor Wt1-null ES cells (GFP+ve) to generate diverse hematopoietic cell types within chimeric fetal livers in similar proportions to the endogenous wild-type host (GFP–ve): (i) Mac-1 (myeloid lineage), (ii) c-Kit (progenitor cells), (iii) Ter-119 (erythroid lineage), and (iv) B220 (lymphoid lineage). Data from the chimera with the highest contribution of GFP+ve Wt1-null ES cells are shown.
Mice carrying a hypomorphic allele of Vegf-a show increased apoptosis of hematopoietic progenitor cells10 reminiscent of the phenotype of Wt1-null EBs. Given the growing body of evidence that VEGF-A is a WT1 target,11-13 we hypothesized that Wt1 may be regulating Vegf-a in EBs and tested whether exogenous Vegf-a protein could restore hematopoiesis in Wt1-null EBs. Exogenous Vegf-a restored close to normal hematopoietic colony-forming potential to Wt1-deficient cells (Figure 2A) by reducing apoptosis of Ter119+ve progenitors lacking Wt1 (Figure 2B-C).
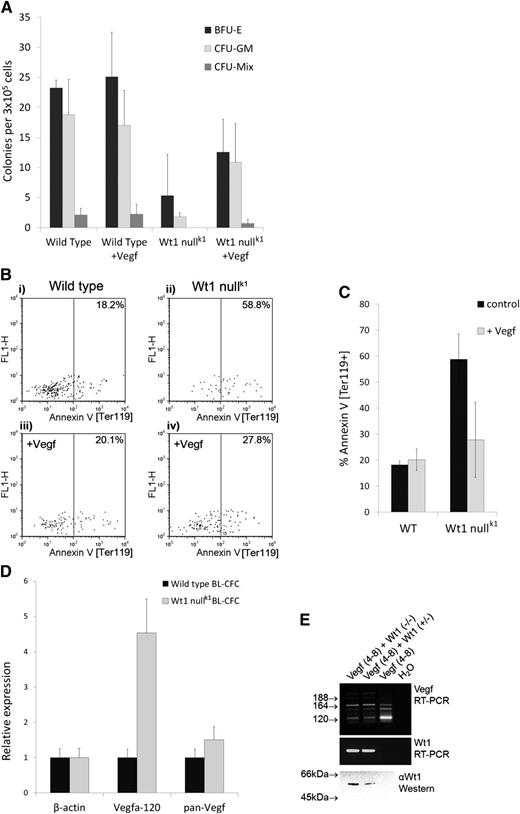
Exogenous Vegf-a164 rescues the hematopoietic impairment of Wt1-null EBs. (A) Hematopoietic colony assay (Stem Cell Technologies) of disaggregated day 8 EBs following Vegf-a164 treatment showing numbers of erythroid (BFU-E), myeloid (CFU-GM), and multipotent (CFU-mix) progenitor cells per 3 × 105 EB cells. Addition of exogenous Vegf-a164 significantly increases hematopoietic potential of Wt1-null EBs (total colony counts; P < .05). The data are the mean of 3 independent replicates, each conducted in triplicate. Bars display standard deviation; significance tested using analysis of variance. (B-C) Exogenous Vegf-a164 blocks apoptosis in Wt1-null EBs. (B) Representative FACS analysis of annexin-V staining of cells within the earliest Ter119+ve population of progenitor cells to emerge following 6 days of differentiation in (i) wild-type control, (ii) Wt1-null control, (iii) wild type + Vegf-a164, and (iv) Wt1 null + Vegf-a164. (C) Corresponding graph to (B) showing a significant reduction in apoptosis in Wt1-null EBs in response to Vegf-a164 over 3 independent assays (P < .05). The numerical data are the mean of independent replicates, tested for significance using a paired Student t test; 2-tailed distribution. (D) WT1 controls the ratio of Vegf-a isoforms in hematopoietic progenitor cells. qRT-PCR analysis of wild-type and Wt1-null BL-CFCs for β-actin, Vegf-a120, and pan–Vegf-a showing overrepresentation of Vegf-a120 in Wt1-null BL-CFCs. (E) Vegf-a splicing assay. RT-PCR analysis of Vegf-a isoforms following transient transfection of Cos-7 cells with a genomic expression construct encompassing Vegf-a exons 4 to 8 (lanes 1-3) cotransfected with Wt1 expression constructs for the “-KTS” isoforms, Wt1(−/−) and Wt1 (+/−), lanes 1 and 2. Primers located in exons 4 and 8 amplify Vegf-a120 (89 bp), Vegf-a164 (221 bp), and Vegf-a189 (293 bp). Nonspecific background bands are also present associated with an artifactual heteroduplex, as previously reported.15 Lower panels show Wt1 expression confirmed by RT-PCR and western blotting using monoclonal antibody 6F-H2 (DAKO).
Exogenous Vegf-a164 rescues the hematopoietic impairment of Wt1-null EBs. (A) Hematopoietic colony assay (Stem Cell Technologies) of disaggregated day 8 EBs following Vegf-a164 treatment showing numbers of erythroid (BFU-E), myeloid (CFU-GM), and multipotent (CFU-mix) progenitor cells per 3 × 105 EB cells. Addition of exogenous Vegf-a164 significantly increases hematopoietic potential of Wt1-null EBs (total colony counts; P < .05). The data are the mean of 3 independent replicates, each conducted in triplicate. Bars display standard deviation; significance tested using analysis of variance. (B-C) Exogenous Vegf-a164 blocks apoptosis in Wt1-null EBs. (B) Representative FACS analysis of annexin-V staining of cells within the earliest Ter119+ve population of progenitor cells to emerge following 6 days of differentiation in (i) wild-type control, (ii) Wt1-null control, (iii) wild type + Vegf-a164, and (iv) Wt1 null + Vegf-a164. (C) Corresponding graph to (B) showing a significant reduction in apoptosis in Wt1-null EBs in response to Vegf-a164 over 3 independent assays (P < .05). The numerical data are the mean of independent replicates, tested for significance using a paired Student t test; 2-tailed distribution. (D) WT1 controls the ratio of Vegf-a isoforms in hematopoietic progenitor cells. qRT-PCR analysis of wild-type and Wt1-null BL-CFCs for β-actin, Vegf-a120, and pan–Vegf-a showing overrepresentation of Vegf-a120 in Wt1-null BL-CFCs. (E) Vegf-a splicing assay. RT-PCR analysis of Vegf-a isoforms following transient transfection of Cos-7 cells with a genomic expression construct encompassing Vegf-a exons 4 to 8 (lanes 1-3) cotransfected with Wt1 expression constructs for the “-KTS” isoforms, Wt1(−/−) and Wt1 (+/−), lanes 1 and 2. Primers located in exons 4 and 8 amplify Vegf-a120 (89 bp), Vegf-a164 (221 bp), and Vegf-a189 (293 bp). Nonspecific background bands are also present associated with an artifactual heteroduplex, as previously reported.15 Lower panels show Wt1 expression confirmed by RT-PCR and western blotting using monoclonal antibody 6F-H2 (DAKO).
In the course of these studies, we observed that Wt1-null EBs contained normal numbers of hemangioblasts as determined by the hemangioblast colony-forming cell (BL-CFC) assay14 (data not shown). Using these BL-CFC colonies as a source of messenger RNA (mRNA) immediately preceding a detectable phenotype in the absence of Wt1, we conducted quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis. Overall levels of Vegf-a mRNA did not differ greatly between wild-type and Wt1-null BL-CFCs; however, qRT-PCR revealed a striking overrepresentation of Vegf-a120 mRNA in Wt1-null BL-CFCs (Figure 2D). This was an unexpected finding given that Wt1 has been shown to upregulate Vegf-a expression12 and suggested that Wt1 may be regulating Vegf-a alternative splicing. To directly test whether Wt1 was capable of regulating Vegf-a alternative splicing, we carried out splicing assays15 on a Vegf-a minigene encompassing the alternatively spliced exons 4 to 8 in their native genomic context, in a heterologous cell type, Cos-7. Consistent with loss of Wt1 function increasing Vegf-a120, transfection of Wt1 promotes exon inclusion and reduces the amount of Vegf-a120 (Figure 2E), indicating that Wt1 is necessary and sufficient for regulating Vegf-a isoforms. The acidic Vegf-a120 isoform is freely diffusible, having a greatly reduced affinity for heparin sulfate proteoglycans, compared with the basic, longer, isoforms.16 Thus, the rescue of Wt1-null EBs by addition of exogenous Vegf-a164 protein is consistent with correction of reduced bioavailability. Such a mechanism reconciles previous, contradictory, findings regarding the role of Wt1 in hematopoiesis, in particular the reduced in vitro colony-forming potential of Wt1-null fetal liver cells that can successfully reconstitute irradiated mice.6 We propose that endogenous Vegf-a, produced by wild-type cells in the host animal, can rescue Wt1-null hematopoietic cells from apoptosis, whereas this factor is lacking in the Stem Cell Technologies colony assay media. Furthermore, our model can explain the report that homozygous Wt1tmT396 mutant EBs show a delay but no reduction in hematopoiesis,8 whereas we see no evidence of delayed hematopoiesis across 18 days of differentiation (supplemental Figure 3). Closer inspection reveals that the CFU-A assay employed in the study of Wt1tmT396 mutant EBs uses a feeder layer containing L929 conditioned medium,8 which has recently been shown to contain >85 ng/mL of VEGF-A,17 notably of a similar order of magnitude to the 10 ng/mL of Vegf-A164 used in the present study. Thus, we would suggest that VEGF-A present in the L929 conditioned medium is affecting a partial rescue of the Wt1 deficiency.
In summary, we show that Wt1-null hematopoietic progenitor cells undergo Vegf-a–dependent apoptosis associated with a shift in Vegf-a isoforms toward Vegf-a120. This provides a feasible explanation for the contradictions in the literature and implicates VEGF-A as a strong candidate target for WT1-mediated splicing regulation. Furthermore, high levels of VEGF-121 (the human equivalent of murine Vegf-a120) have recently been identified as an independent prognostic factor associated with poor survival in AML.18 Given that WT1 mutation also correlates with poor prognosis in AML,2-4 and the effect of Wt1 loss of function can be abrogated by exogenous Vegf-a164, the WT1-VEGF pathway we describe potentially represents a novel therapeutic target in poor prognosis AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lorraine Eley, Eilidh Mackenzie, Simon Fitch, and Lee Spraggon for their technical assistance and Ian Wilson for statistical advice.
This work was supported by grants from CLIC-Sargent UK (T.J.C.); the BBSRC, UK, and the Leukaemia Research Fund, UK (C.G.M.); and Ministero Salute-Ricerca Oncologica-RECAM-2006-353005 and PRIN 2007-prot.2007EN8F7T-004 (I.P. and M.G.).
Authorship
Contribution: T.J.C. and C.G.M. performed most of the experiments and wrote the manuscript; N.S. carried out lentiviral transductions; I.P. carried out splicing assays; M.G. supervised I.P. and facilitated the visit of I.P. to the Miles laboratory; and C.G.M. conceived the study, obtained the funding, and directed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Colin G. Miles, Newcastle University Institute of Genetic Medicine, International Centre for Life, Central Parkway, Newcastle, NE1 3BZ, United Kingdom; e-mail: c.g.miles@ncl.ac.uk.

![Figure 1. Hematopoiesis is significantly perturbed in Wt1-deficient EBs. (A) Representative FACS plots of disaggregated wild-type and Wt1-nullk1 EBs stained at (i) day 6 of differentiation for CD41/c-Kit (n = 7; P < .0005), (ii) day 6 for Ter119 (n = 5; P < .01), and (iii) day 8 for Mac-1 (n = 5; P < .0005), showing fewer hematopoietic progenitor cells, mature erythroid cells, and myeloid cells in Wt1-null EBs. The numerical data are the mean of independent replicates, tested for significance using Student t test; 2-tailed distribution assuming unequal variance. P values represent both Wt1-null clones vs wild type. (B) Hematopoietic colony assay (Stem Cell Technologies) of disaggregated day 8 EBs showing numbers of erythroid (BFU-E), myeloid (CFU-GM), and multipotent (CFU-mix) progenitor cells per 3 × 105 EB cells. Both Wt1-null clones individually show a significant reduction in colony-forming potential (P < .005), including all individual colony types (P < .05), compared with wild type. The data are the mean of independent replicates, each conducted in triplicate. Bars display standard deviation; significance tested using analysis of variance (n [wild type vs K1] = 4; n [wild type vs K46] = 3). (C) Hematopoietic progenitor cells lacking Wt1 show elevated levels of apoptosis compared with wild type. Representative FACS plots for (i) annexin-V staining of cells gated for Ter119+ve expression showing increased levels of apoptosis in Wt1-null EBs compared with wild type (n = 5; P < .02) and (ii) annexin-V staining of unfractionated, disaggregated wild-type and Wt1-null EBs showing that the increased apoptosis of hematopoietic progenitors is not a general feature of all EB cells lacking Wt1 (n = 5; P > .05). The numerical data are the mean of independent replicates, tested for significance using Student t test; 2-tailed distribution assuming unequal variance. P values represent both Wt1-null clones vs wild type. (D) FACS analysis of e15.5 fetal livers from chimeric embryos showing capacity of donor Wt1-null ES cells (GFP+ve) to generate diverse hematopoietic cell types within chimeric fetal livers in similar proportions to the endogenous wild-type host (GFP–ve): (i) Mac-1 (myeloid lineage), (ii) c-Kit (progenitor cells), (iii) Ter-119 (erythroid lineage), and (iv) B220 (lymphoid lineage). Data from the chimera with the highest contribution of GFP+ve Wt1-null ES cells are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/2/10.1182_blood-2012-11-466086/4/m_188f1.jpeg?Expires=1767714781&Signature=PCuUVUjoWcWV-XqVd5K2CZAg7CZbMlr3~dX7xcN7AIm7hhtCWLljmGBgII68uU9utgpcCfobPdoDBILmNdhni47XfEBj4~fZP8zKqLVWK5iJLuE52JTF24zKnQrYcy7IBihVr-RLU4zISxIGybyxhtrdjYEwrt-VQc9g-yC-iXiWalTo2WwdIZVP0ooum1Zv03W4ut6vvMrtSKfa52q4fTduki4mxR9SNgXpTdxxbDH4~YiKq~sZLgH-rtC6KdjDFcNt5INU05lMNYH1QfJ7RRo057WaIm~Q-FXhAsVw69M-VebD9iCn0SsP2cUzvS6UHXt3WT5m5nOVVCBJP5DjVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal