To the editor:
A 54-year-old man with no significant past medical history presented with skin rash. Complete blood count showed a white blood cell count of 64 × 109/L (63% neutrophils, 2% eosinophils), hemoglobin 14 g/dL, and platelet count 166 × 109/L. Bone marrow evaluation showed a hypercellular marrow with marked granulocytic hyperplasia (Figure 1). There was no increase in marrow eosinophils or fibrosis. No dysplasia was identified. Conventional cytogenetics showed constitutional inv(9). Testing for BCR-ABL rearrangement and JAK2V617F mutation was negative. The patient was diagnosed with chronic neutrophilic leukemia (CNL) and treatment with hydroxyurea was initiated. Subsequently, array comparative genomic hybridization demonstrated monoallelic interstitial deletion of chromosome 4q12. This was confirmed by interphase fluorescence in situ hybridization (FISH) analysis using a probe set that detects loss of CHIC2 (surrogate marker of the fusion of the factor interacting with PAP [Fip1]-like 1 [FIP1L1] gene to the platelet derived growth factor receptor-α [PDGFRA] gene) and by reverse-transcription polymerase chain reaction. The diagnosis was revised to myeloproliferative neoplasm with FIP1L1-PDGFRA fusion Imatinib (100 mg daily) was prescribed. Hydroxyurea was discontinued. The white blood cell count normalized within 2 weeks and follow-up testing for FIP1L1-PDGFRA fusion by FISH at 3 months was negative. The patient continues to be treated with imatinib 100 mg daily for >1 year with no recurrence of skin rash or leukocytosis.
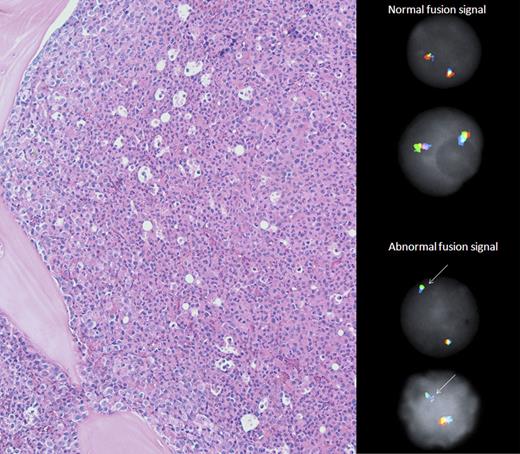
Bone marrow morphology and CHIC2 FISH. The bone marrow biopsy (left panel) demonstrated hypercellularity and marked predominance of maturing neutrophilic granulocytes. Megakaryocytes were decreased, and no features of myelofibrosis or myelodysplasia were present. Notably, eosinophils and blasts were rare. By fluorescence in situ hybridization (right panel) using a tricolor probe set (Abbott Molecular), normal cells had two intact tricolor (green/orange/aqua) fusion signals whereas abnormal cells (90% in this sample) with monoallelic loss of CHIC2 (orange pseudocolor) had one green/aqua fusion signal.
Bone marrow morphology and CHIC2 FISH. The bone marrow biopsy (left panel) demonstrated hypercellularity and marked predominance of maturing neutrophilic granulocytes. Megakaryocytes were decreased, and no features of myelofibrosis or myelodysplasia were present. Notably, eosinophils and blasts were rare. By fluorescence in situ hybridization (right panel) using a tricolor probe set (Abbott Molecular), normal cells had two intact tricolor (green/orange/aqua) fusion signals whereas abnormal cells (90% in this sample) with monoallelic loss of CHIC2 (orange pseudocolor) had one green/aqua fusion signal.
Imatinib is approved for treatment of patients with FIP1L1-PDGFRA fusion-positive myeloid neoplasms.1 These patients typically present with peripheral blood eosinophilia. Cools et al2 described this entity as an interstitial deletion of chromosome 4q12 that leads to the juxtaposition of the FIP1L1 gene to the PDGFRA gene. The resultant fusion product, FIP1L1-PDGFRA, results in constitutive activation of the tyrosine kinase PDGFRA and is amenable to therapy with imatinib. Baccarani et al3 reported achievement of complete hematologic response with imatinib in all patients with FIP1L1-PDGFRA fusion and the responses were durable.4
Our patient presented with characteristic diagnostic features of patients with CNL (neutrophilic leukocytosis, hypercellular bone marrow with granulocytic hyperplasia, and absence of dysplasia).5 Although the patient did not present with eosinophilia, FIP1L1-PDGFRA fusion was tested because of the presence of skin rash and, remarkably, the test was positive. To the best of our knowledge, this is the first case of a FIP1L1-PDGFRA fusion without eosinophilia that responded to tyrosine kinase inhibitor therapy. There is a recent report of FIP1L1-PDGFRA fusion without eosinophilia in a patient with monoclonal gammopathy; however, the response to imatinib was not reported.6 Imatinib response in a patient with CNL has also been reported previously; however, the molecular mechanism for the response was not elucidated.7 The diagnosis could easily have been missed in our patient because he did not present with eosinophilia. Establishing the correct diagnosis significantly altered the treatment management because FIP1L1-PDGFRA fusion is extremely sensitive to imatinib. The 2008 World Health Organization classification lists “myeloid neoplasms associated with PDGFRA rearrangement” under “myeloid neoplasms associated with eosinophilia and abnormalities of PDGFRA,” indicative of a strong emphasis on the presence of eosinophilia.8 This is a report of a single patient, but because of the enormous therapeutic implications, we recommend that evaluation for FIP1L1-PDGFRA fusion should be considered for all patients with nonclassical myeloproliferative neoplasms. We evaluated 7 additional CNL patients who had stored samples for FIP1L1-PDGFRA fusion by FISH; however, all were negative. Recently, activating CSF3R mutations were identified in majority of patients with CNL.9,10 It is possible that CNL patients without CSF3R mutations (17% to 41% of cases) likely have alternate molecular pathogenic lesions leading to constitutive activation of other tyrosine kinases such as PDGFRA.
Authorship
Contribution: N.J., J.D.K., N.P., P.K., H.K., and S.V. collected data, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Department of Leukemia, Clinical Research Center for Myeloproliferative Neoplasms, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX; e-mail: sverstov@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal