Key Points
Donor and/or recipient CMV seropositivity is still associated with an adverse prognosis in de novo acute leukemia patients after allo-SCT.
Abstract
We analyzed the prognostic impact of donor and recipient cytomegalovirus (CMV) serostatus in 16 628 de novo acute leukemia patients after allogeneic stem cell transplantation (allo-SCT). Compared with CMV-seronegative recipients who underwent allograft from a CMV-seronegative donor, cases of CMV seropositivity of the donor and/or the recipient showed a significantly decreased 2-year leukemia-free survival (44% vs 49%, P < .001) and overall survival (50% vs 56%, P < .001), and increased nonrelapse mortality (23% vs 20%, P < .001). Both groups showed a comparable relapse incidence and 2-year probability of graft-versus-host disease. The negative prognostic effects of CMV seropositivity of the donor and/or the recipient (vs CMV seronegativity of both) were significantly stronger for acute lymphoblastic leukemia (ALL) than for acute myeloid leukemia (AML), resulting in a markedly reduced 2-year overall survival (46% vs 55% for ALL compared with 52% vs 56% for AML). The important prognostic impact of donor/recipient CMV serostatus remained in a multivariate Cox regression analysis including the other prognostic variables. We conclude that donor and/or recipient CMV seropositivity is still associated with an adverse prognosis in de novo acute leukemia patients after allo-SCT despite the implementation of sophisticated strategies for prophylaxis, monitoring, and (preemptive) treatment of CMV.
Introduction
Monitoring of cytomegalovirus (CMV) (eg, using polymerase chain reaction [PCR]) and its (preemptive) treatment (eg, ganciclovir) have been implemented in standard algorithms in patients after allogeneic stem cell transplantation (allo-SCT) in the 1990s and resulted in a dramatically reduced frequency of CMV disease, from 20% to 30% to <5% in recent years.1-3 However, as much as 80% of patients still develop CMV infection (ie, reactivation) after allo-SCT, with recipient CMV seropositivity (R-CMV+), allografting from a donor other than a human leukocyte antigen (HLA)-identical sibling, and the use of in vivo T-cell depletion (eg, with alemtuzumab) being the most important risk factors.3-6 Despite this, the prognostic significance of (asymptomatic) CMV infection remains controversial in patients after allo-SCT. A significantly reduced relapse incidence (RI) in acute myeloid leukemia (AML) patients after allo-SCT with vs without early CMV replication has been reported recently.7-9 These unexpected clinical observations suggesting a “virus-vs-leukemia” effect were further supported by in vitro studies, which suggested that CMV might trigger natural killer cell–mediated antileukemia effects.10 In contrast, other investigators found a negative prognostic influence of CMV infection after allo-SCT and attributed these observations to side effects of antiviral agents used for preemptive CMV treatment, acute graft-versus-host disease (GVHD), which may be associated with CMV infection, or a high risk for late CMV disease.1,11
Currently, the prognostic impact of R-CMV+ as a major risk factor for CMV infection and disease likewise remains only partially understood.12 Several studies reported a negative prognostic impact of R-CMV+ vs recipient CMV seronegativity (R-CMV–) with an absolute decline in overall survival (OS) of as much as 40%.12-15 However, these studies were rather heterogeneous with regard to the underlying disease (eg, acute and chronic leukemia, aplastic anemia) and, importantly, were not fully restricted to the era of CMV monitoring and preemptive treatment of CMV infection as routine procedures. Data with respect to the prognostic impact of the donor CMV serostatus are even more scarce and conflicting in patients after allo-SCT, although the majority of transplant centers currently prefer a CMV-seronegative donor for a CMV-seronegative recipient and a CMV-seropositive donor for a CMV-seropositive recipient.12,15,16 We wanted to evaluate whether donor CMV seropositivity (D-CMV+) and/or R-CMV+ was still associated with an adverse prognosis despite the implementation in recent years of sophisticated strategies for prophylaxis, monitoring, and (preemptive) treatment of CMV. For this purpose, we analyzed 16 628 patients with de novo acute leukemia after allo-SCT who were documented in the database of the European Blood and Marrow Transplantation (EBMT) group.
Patients and methods
Study design, data collection, and criteria for selection
Adult patients (≥18 years) with de novo acute lymphoblastic leukemia (ALL) or AML who underwent allo-SCT between 1998 and 2009 and were documented in the EBMT database were included in this retrospective multicenter study. Only patients with full data available on type of conditioning (reduced intensity conditioning [RIC] vs myeloablative conditioning [MAC]), remission status of the underlying malignancy at allo-SCT, source of stem cells (ie, peripheral blood [PB] vs bone marrow [BM]), and both donor and recipient CMV serostatus were analyzed (n = 16 628). This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Acute Leukemia Working Party (ALWP) of the EBMT group.
Statistical analyses and definitions
The primary endpoints encompassed the probabilities of leukemia-free survival (LFS), RI, nonrelapse mortality (NRM), and OS. Secondary end points were acute and chronic GVHD. LFS was considered to be survival with no evidence of relapse or progression. Relapse was defined as the presence of >5% BM blasts and/or reappearance of the underlying disease. NRM was defined as death without evidence of relapse or progression. OS was defined as the time from allo-SCT to death, regardless of the cause. Acute and chronic GVHD were graded according to previously published criteria.17 Cytogenetic risk groups were defined as previously suggested (good risk = presence of inversion 16, t(8;21), or t(15;17); poor risk = complex (≥3) cytogenetic abnormalities, deletion 5, deletion 7, t(6;9), 11q-abnormalities, or the presence of t(9;22) or t(4;11) for ALL; and intermediate risk = normal karyotype or other cytogenetic abnormalities).18
Factors considered were diagnosis (ALL or AML), the patient’s age at transplantation, donor/recipient sex matching (female donor to male recipient vs other), type of donor (HLA-matched sibling vs other donor types), type of conditioning (RIC vs MAC), status at time of transplantation (ie, first complete remission [CR] vs other), source of stem cells (PB vs BM), year of transplantation and CMV serostatus of recipient (R-CMV– vs R-CMV+), donor (ie, donor CMV seronegativity [D-CMV–] vs D-CMV+), and donor/recipient combinations (D-CMV–/R-CMV–, D-CMV+/R-CMV–, D-CMV–/R-CMV+, D-CMV+/R-CMV+). Additional variables such as cytogenetic risk groups, different types of T-cell depletion, center size, and donor/recipient HLA matching were also included in a more detailed analysis (supplemental data). For all prognostic analyses, continuous variables were categorized, and the median was used as a cutoff point. Comparisons for categorical variables were done using the Fisher’s exact test or the χ2 test. Cumulative incidence functions (CIF) were used to estimate RI and NRM in a competing risks setting, because death and relapse compete with each other.19 To study chronic GVHD, we considered death to be a competing event. Specific causes of deaths were also estimated using cumulative incidence curves, considering death from any other cause as a competing event. Probabilities of LFS and OS were calculated using the Kaplan-Meier estimates. All probabilities are given in percentage. Univariate analyses were performed using Gray’s test for CIF and the log-rank test for LFS and OS. All factors studied were included in the Cox proportional hazards model for all end points under the study except acute GVHD, for which logistic regression was used.20 All tests were two-sided, with the type I error rate fixed at .05. To take subgroup comparisons into account, the Bonferroni correction was applied, with an α value < .016 for 3 subgroup analyses. Statistical analyses were performed with the SPSS 19 (SPSS Inc./IBM, Armonk, NY) and R 2.13.2 (R Development Core Team, Vienna, Austria) software packages.
Results
Patients
16 628 patients with de novo acute leukemia who underwent allo-SCT between 1998 and 2009 and were reported in the registry of the ALWP of EBMT were included in this study. Patient and transplant characteristics are summarized in Table 1 (for different donor types, see supplemental Table 1). D-CMV–/R-CMV– status was recorded in 4287 cases (26%), and in 12 341 cases (74%) D-CMV+ and/or R-CMV+ was present. CMV-seropositive recipients (n = 10 638) were preferentially allografted from a CMV-seropositive donor (n = 7008, 66%), likely reflecting the current strategy of many transplant centers to match recipient and donor according to their CMV serostatus.12,16
Patient and transplant characteristics
| Parameter . | D-CMV–/R-CMV– (n = 4287) . | D-CMV+/R-CMV– (n = 1703) . | D-CMV–/R-CMV+ (n = 3630) . | D-CMV+/R-CMV+ (n = 7008) . |
|---|---|---|---|---|
| Underlying malignancy* | ||||
| ALL | 1454 (34%) | 597 (35%) | 1119 (31%) | 1988 (28%) |
| AML | 2833 (66%) | 1106 (65%) | 2511 (69%) | 5020 (72%) |
| Male sex (recipient)* | 2451 (57%) | 1052 (62%) | 1846 (51%) | 3732 (53%) |
| Recipient’s age, y* | 39 (18-76) | 41 (18-75) | 43 (18-76) | 43 (18-77) |
| Male sex (donor)* | 2782 (65%) | 941 (55%) | 2420 (67%) | 3939 (56%) |
| Donor/recipient sex matching* | ||||
| Female to male | 767 (18%) | 443 (26%) | 560 (15%) | 1491 (21%) |
| White blood count at diagnosis, × 109/L† | 14.3 (0.1-879) | 13.3 (0.1-850) | 14.6 (0.2-800) | 14.1 (0.1-790) |
| Donor type*‡ | ||||
| HLA-identical sibling or syngeneic | 2090 (49%) | 856 (50%) | 1403 (39%) | 4504 (64%) |
| Other | 2197 (51%) | 847 (50%) | 2227 (61%) | 2504 (36%) |
| Conditioning* | ||||
| MAC | 3320 (77%) | 1274 (75%) | 2621 (72%) | 5244 (75%) |
| RIC | 967 (23%) | 429 (25%) | 1009 (28%) | 1764 (25%) |
| Cytogenetic risk group§ | ||||
| Good | 183 (9%) | 76 (9%) | 163 (9%) | 328 (10%) |
| Intermediate | 1136 (53%) | 455 (51%) | 941 (54%) | 1847 (56%) |
| Poor | 807 (38%) | 365 (41%) | 653 (37%) | 1149 (35%) |
| Remission status at allo-SCT§ | ||||
| 1. CR | 2704 (63%) | 1064 (63%) | 2132 (59%) | 4309 (62%) |
| 2. CR | 831 (19%) | 320 (19%) | 728 (20%) | 1367 (20%) |
| Active disease | 752 (18%) | 319 (19%) | 770 (21%) | 1332 (19%) |
| Stem cell source* | ||||
| BM | 1307 (31%) | 501 (29%) | 990 (27%) | 1656 (24%) |
| PB | 2980 (70%) | 1202 (71%) | 2640 (73%) | 5352 (76%) |
| TCD* | ||||
| No | 2265 (53%) | 910 (53%) | 1725 (48%) | 4214 (60%) |
| ATG | 1138 (27%) | 451 (27%) | 1263 (35%) | 1663 (24%) |
| Alemtuzumab | 499 (12%) | 177 (10%) | 380 (11%) | 491 (7%) |
| Ex vivo | 385 (9%) | 165 (10%) | 262 (7%) | 640 (9%) |
| Number of transplants per center* | 104 (1-339) | 105 (1-339) | 101 (1-339) | 90 (1-339) |
| Year of allo-SCT* | May 2006 (1998-2009) | February 2006 (1998-2009) | October 2006 (1998-2009) | Sept. 2006 (1998-2009) |
| Follow-up, mo§ | 26 (0.1-157) | 27 (0.1-156) | 24 (0.6-153) | 24 (0.5-154) |
| Parameter . | D-CMV–/R-CMV– (n = 4287) . | D-CMV+/R-CMV– (n = 1703) . | D-CMV–/R-CMV+ (n = 3630) . | D-CMV+/R-CMV+ (n = 7008) . |
|---|---|---|---|---|
| Underlying malignancy* | ||||
| ALL | 1454 (34%) | 597 (35%) | 1119 (31%) | 1988 (28%) |
| AML | 2833 (66%) | 1106 (65%) | 2511 (69%) | 5020 (72%) |
| Male sex (recipient)* | 2451 (57%) | 1052 (62%) | 1846 (51%) | 3732 (53%) |
| Recipient’s age, y* | 39 (18-76) | 41 (18-75) | 43 (18-76) | 43 (18-77) |
| Male sex (donor)* | 2782 (65%) | 941 (55%) | 2420 (67%) | 3939 (56%) |
| Donor/recipient sex matching* | ||||
| Female to male | 767 (18%) | 443 (26%) | 560 (15%) | 1491 (21%) |
| White blood count at diagnosis, × 109/L† | 14.3 (0.1-879) | 13.3 (0.1-850) | 14.6 (0.2-800) | 14.1 (0.1-790) |
| Donor type*‡ | ||||
| HLA-identical sibling or syngeneic | 2090 (49%) | 856 (50%) | 1403 (39%) | 4504 (64%) |
| Other | 2197 (51%) | 847 (50%) | 2227 (61%) | 2504 (36%) |
| Conditioning* | ||||
| MAC | 3320 (77%) | 1274 (75%) | 2621 (72%) | 5244 (75%) |
| RIC | 967 (23%) | 429 (25%) | 1009 (28%) | 1764 (25%) |
| Cytogenetic risk group§ | ||||
| Good | 183 (9%) | 76 (9%) | 163 (9%) | 328 (10%) |
| Intermediate | 1136 (53%) | 455 (51%) | 941 (54%) | 1847 (56%) |
| Poor | 807 (38%) | 365 (41%) | 653 (37%) | 1149 (35%) |
| Remission status at allo-SCT§ | ||||
| 1. CR | 2704 (63%) | 1064 (63%) | 2132 (59%) | 4309 (62%) |
| 2. CR | 831 (19%) | 320 (19%) | 728 (20%) | 1367 (20%) |
| Active disease | 752 (18%) | 319 (19%) | 770 (21%) | 1332 (19%) |
| Stem cell source* | ||||
| BM | 1307 (31%) | 501 (29%) | 990 (27%) | 1656 (24%) |
| PB | 2980 (70%) | 1202 (71%) | 2640 (73%) | 5352 (76%) |
| TCD* | ||||
| No | 2265 (53%) | 910 (53%) | 1725 (48%) | 4214 (60%) |
| ATG | 1138 (27%) | 451 (27%) | 1263 (35%) | 1663 (24%) |
| Alemtuzumab | 499 (12%) | 177 (10%) | 380 (11%) | 491 (7%) |
| Ex vivo | 385 (9%) | 165 (10%) | 262 (7%) | 640 (9%) |
| Number of transplants per center* | 104 (1-339) | 105 (1-339) | 101 (1-339) | 90 (1-339) |
| Year of allo-SCT* | May 2006 (1998-2009) | February 2006 (1998-2009) | October 2006 (1998-2009) | Sept. 2006 (1998-2009) |
| Follow-up, mo§ | 26 (0.1-157) | 27 (0.1-156) | 24 (0.6-153) | 24 (0.5-154) |
Shown are numbers of patients (%) or medians (range). Data are not available for missing patients from the total cohort.
ATG, antithymocyte globulin; TCD, T-cell depletion.
P (global) < .0001.
Missing data in 2076, 765, 1939, and 3625 patients, respectively.
Different donor types are shown in detail in supplemental Table 1.
P < .05.
Survival and relapse of the underlying malignancy (univariate analyses)
LFS and OS estimates were 45% and 51% at 2 years, 39% and 43% at 5 years, and 35% and 38% at 10 years after allo-SCT. Compared with CMV-seronegative recipients who underwent allo-SCT from a CMV-seronegative donor, cases with D-CMV+ and/or R-CMV+ showed a significantly decreased 2-year LFS and OS. These observations correlated primarily with an increased NRM, whereas RI was slightly increased (P = .08; Table 2). Furthermore, D-CMV+ vs D-CMV– was associated with a significantly reduced LFS (P = .001) and OS (P < .0001) among CMV-seronegative recipients. In turn, CMV-seropositive recipients with a CMV-seropositive donor showed a significantly (P = .04) superior OS than CMV-seropositive recipients with a CMV-seronegative donor, whereas the LFS was comparable between both groups (P = .24; Table 2).
Impact of CMV serostatus on LFS, RI, NRM, OS, chronic GVHD, and neutrophil engraftment
| CMV serostatus . | LFS . | RI . | NRM . | OS . | cGVHD . | NEG . |
|---|---|---|---|---|---|---|
| Total (n = 16 628) | 45 | 32 | 22 | 51 | 45 | 97 |
| D-CMV–/R-CMV– | 49 | 31 | 20 | 56 | 45 | 97 |
| D-CMV+/R-CMV– | 44 | 34 | 22 | 49 | 47 | 96 |
| D-CMV–/R-CMV+ | 43 | 31 | 25 | 49 | 44 | 96 |
| D-CMV+/R-CMV+ | 45 | 33 | 23 | 51 | 44 | 96 |
| P | < .001 | .11 | < .001 | < .001 | .63 | < .001 |
| D-CMV–/R-CMV– | 49 | 31 | 20 | 56 | 45 | 97 |
| Other combination | 44 | 32 | 23 | 50 | 45 | 96 |
| P | < .001 | .08 | < .001 | < .001 | .6 | .08 |
| CMV serostatus . | LFS . | RI . | NRM . | OS . | cGVHD . | NEG . |
|---|---|---|---|---|---|---|
| Total (n = 16 628) | 45 | 32 | 22 | 51 | 45 | 97 |
| D-CMV–/R-CMV– | 49 | 31 | 20 | 56 | 45 | 97 |
| D-CMV+/R-CMV– | 44 | 34 | 22 | 49 | 47 | 96 |
| D-CMV–/R-CMV+ | 43 | 31 | 25 | 49 | 44 | 96 |
| D-CMV+/R-CMV+ | 45 | 33 | 23 | 51 | 44 | 96 |
| P | < .001 | .11 | < .001 | < .001 | .63 | < .001 |
| D-CMV–/R-CMV– | 49 | 31 | 20 | 56 | 45 | 97 |
| Other combination | 44 | 32 | 23 | 50 | 45 | 96 |
| P | < .001 | .08 | < .001 | < .001 | .6 | .08 |
Two-year probabilities (%; standard error ± 1 for all parameters) are shown.
cGVHD, chronic GVHD; NEG, neutrophil engraftment.
When analyzing the different causes of death, D-CMV–/R-CMV– (vs D-CMV+ and/or R-CMV+) was associated with a significantly (P = .002) decreased 2-year cumulative incidence of death owing to infection (7.4% vs 9.2%; supplemental Figure 1), whereas death caused by interstitial pneumonitis was only in tendency (P = .02) decreased (0.9% vs 1.4%). In contrast, CMV-seronegative recipients with a CMV-seronegative donor showed a trend (P = .02) toward an increased 2-year probability of death as a result of secondary malignancies (0.4% vs 0.1%, supplemental Table 2).
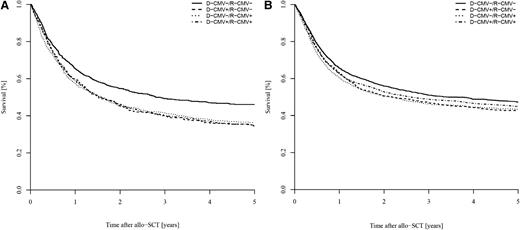
When studying possible interactions between CMV serostatus (D-CMV–/R-CMV– vs D-CMV+ and/or R-CMV+) and all other factors, we found significant interaction between diagnosis and CMV serostatus with regard to LFS (P = .03) and OS (P = .01), reflecting the higher impact of the CMV serostatus in ALL vs AML. With this background, we analyzed patients with ALL and AML separately and found that the negative impact of D-CMV+ and/or R-CMV+ on LFS and OS remained in both cohorts (supplemental Table 3). However, this negative prognostic effect was more pronounced in ALL than in AML, resulting in a markedly decreased 2-year OS (46% and 55% in ALL vs 52% and 56% in AML, Figure 1). ALL patients with D-CMV+ and/or R-CMV+ likewise had a significantly lower LFS (but both higher RI and NRM) than ALL patients with D-CMV–/R-CMV–. In contrast, the reduction of 2-year LFS and OS in AML patients with D-CMV+ and/or R-CMV+ (vs D-CMV–/R-CMV–) was caused by an increased NRM but not an increased RI (supplemental Table 3).
Impact of donor/recipient CMV serostatus on OS. Impact in (A) ALL vs (B) AML.
Engraftment and GVHD (univariate analyses)
In total, 15 804 of 16 386 evaluable patients (96%) had a timely and stable neutrophil engraftment that occurred at a median of 16 days (range, 0-121) after allo-SCT (supplemental Table 4). Primary and secondary graft failure was documented in 496 (3%) and 86 (1%) patients, respectively. In analyzing the whole cohort, we found that the donor/recipient CMV serostatus had no significant impact on the frequency of primary or secondary graft failure or on the median time to neutrophil engraftment (supplemental Table 4). However, D-CMV–/R-CMV– (vs D-CMV+ and/or R-CMV+) was associated with a significantly increased 2-year cumulative incidence of neutrophil engraftment among ALL patients but not within the group of AML recipients (supplemental Table 3).
Acute GVHD (II°-IV°) was documented in 4652 patients (29%). It developed in 30% of CMV-seronegative recipients with a CMV-seronegative donor and in 29% of cases with D-CMV+ and/or R-CMV+ (P = .06). However, in comparing CMV-seronegative vs CMV-seropositive recipients, we found the incidence of II°-IV° acute GVHD was significantly (P < .001) decreased if the recipient was CMV-seropositive (28% vs 31%, supplemental Table 4). Chronic GVHD was observed in 5202 of 13 474 evaluable patients (39%), and its 2-year cumulative incidence did not differ significantly when comparing R-CMV+ vs R-CMV– (44% vs 45%) or D-CMV–/R-CMV– vs D-CMV+ and/or R-CMV+ (Table 2).
Multivariate analyses for outcome
We also analyzed the impact of donor/recipient CMV serostatus on LFS, RI, NRM, OS, neutrophil engraftment, and GVHD in a multivariate Cox regression model that included different patient and transplant characteristics (Table 3 and supplemental Table 5). A diagnosis of AML vs ALL, younger age, use of an HLA-identical sibling donor, cytogenetic good risk, transplantation in first CR, allo-SCT after the year 2004, and a large center size (>50 transplants performed per year) were all associated with a significant superior LFS and OS in multivariate analysis (Table 3 and supplemental Table 5). The negative prognostic impact of D-CMV+ and/or R-CMV+ (vs D-CMV–/R-CMV–) remained in this model with a significantly inferior LFS and OS. As in the univariate analyses, these observations were mainly correlated with an increased NRM and, to a lesser extent, with an increased RI. The CMV serostatus had no significant impact on neutrophil engraftment or GVHD in this multivariate Cox regression model. To obtain more insight into the impact of the CMV serostatus in distinct risk groups, we performed multivariate Cox regression analyses separately for ALL vs AML and CMV-seronegative vs CMV-seropositive recipients, with inclusion of additional variables (eg, cytogenetic risk groups, different types of T-cell depletion, center size; supplemental Table 5). These analyses confirmed the findings of univariate analyses with a significantly reduced OS for patients with D-CMV+ and/or R-CMV+ (vs patients with D-CMV–/R-CMV–) for both ALL and AML. As was found in the univariate analyses, these effects were stronger for ALL than for AML. Finally, we found that D-CMV+ had a significant negative prognostic impact among CMV-seronegative recipients, but not within the group of CMV-seropositive recipients in multivariate analysis (supplemental Table 5).
Impact of different patient and transplant characteristics on LFS, RI, NRM, OS, neutrophil engraftment, and GVHD in multivariate Cox regression analysis
| Parameter . | LFS . | RI . | NRM . | OS . | NEG . | aGVHD . | cGVHD . |
|---|---|---|---|---|---|---|---|
| ALL vs AML | 1.33 (1.27-1.39)† | 1.31 (1.23-1.40)† | 1.35 (1.25-1.45)† | 1.29 (1.22-1.35)† | 0.92 (0.89-0.96)† | 1.21 (1.12-1.30)† | 1.05 (0.99-1.12) |
| Age at least 42 y | 1.28 (1.22-1.34)† | 1.08 (1.01-1.15)‡ | 1.59 (1.47-1.70)† | 1.35 (1.28-1.41)† | 0.96 (0.92-0.99)‡ | 1.03 (0.95-1.11) | 1.16 (1.09-1.23)† |
| Female donor to male recipient vs other combination | 1.02 (0.97-1.08) | 0.95 (0.89-1.02) | 1.13 (1.04-1.22)‡ | 1.05 (0.99-1.11) | 0.92 (0.89-0.96)† | 1.12 (1.03-1.22)‡ | 1.28 (1.20-1.37)† |
| HLA-identical sibling vs other donor type | 0.89 (0.85-0.93)† | 1.10 (1.04-1.17)‡ | 0.66 (0.61-0.71)† | 0.83 (0.79-0.87)† | 1.20 (1.16-1.24)† | 0.82 (0.76-0.88)† | 0.98 (0.93-1.04) |
| RIC vs MAC | 1.04 (0.99-1.10) | 1.28 (1.20-1.38)† | 0.77 (0.71-0.85)† | 0.99 (0.93-1.04) | 0.97 (0.93-1.01) | 0.77 (0.70-0.84)† | 0.90 (0.84-0.97)‡ |
| CR vs other disease status | 0.50 (0.48-0.52)† | 0.45 (0.42-0.47)† | 0.58 (0.54-0.62)† | 0.49 (0.47-0.51)† | 1.05 (1.02-1.09)‡ | 0.90 (0.84-0.97)‡ | 0.91 (0.86-0.97)‡ |
| PB vs BM | 1.09 (1.04-1.15)‡ | 1.10 (1.03-1.18)‡ | 1.08 (1.00-1.17)‡ | 1.09 (1.04-1.15)‡ | 1.89 (1.82-1.97)† | 0.84 (0.78-0.91)† | 1.40 (1.31-1.50)† |
| Allo-SCT after the year 2004 | 0.89 (0.85-0.93)† | 0.95 (0.90-1.01) | 0.81 (0.75-0.87)† | 0.88 (0.84-0.92)† | 0.94 (0.91-0.97)‡ | 0.90 (0.83-0.97)‡ | 0.98 (0.93-1.04) |
| D-CMV+/R-CMV– vs D-CMV–/R-CMV– | 1.12 (1.04-1.21)‡ | 1.12 (1.01-1.24)‡ | 1.13 (1.00-1.28)‡ | 1.16 (1.07-1.26)‡ | 1.03 (0.97-1.10) | 1.10 (0.97-1.24) | 1.05 (0.95-1.15) |
| D-CMV–/R-CMV+ vs D-CMV–/R-CMV– | 1.10 (1.04-1.17)‡ | 1.03 (0.95-1.12) | 1.20 (1.09-1.32)‡ | 1.14 (1.07-1.21)‡ | 0.99 (0.94-1.03) | 0.97 (0.87-1.07) | 1.01 (0.93-1.10) |
| D-CMV+/R-CMV+ vs D-CMV–/R-CMV– | 1.13 (1.07-1.20)† | 1.08 (1.01-1.16)‡ | 1.21 (1.11-1.32)† | 1.15 (1.09-1.22)† | 1.00 (0.96-1.04) | 0.92 (0.85-1.01) | 0.98 (0.92-1.05) |
| D-CMV+/R-CMV+ vs D-CMV–/R-CMV+* | 0.97 (0.92-1.03) | 1.05 (0.97-1.13) | 1.00 (0.92-1.09) | 0.99 (0.93-1.05) | 0.99 (0.95-1.03) | 1.05 (0.95-1.15) | 1.03 (0.96-1.11) |
| D-CMV–/R-CMV– vs all other* | 0.89 (0.85-0.94)† | 0.93 (0.87-0.99)‡ | 0.84 (0.77-0.90)† | 0.87 (0.83-0.92)† | 1.00 (0.97-1.04) | 1.04 (0.96-1.13) | 1.00 (0.94-1.06) |
| Parameter . | LFS . | RI . | NRM . | OS . | NEG . | aGVHD . | cGVHD . |
|---|---|---|---|---|---|---|---|
| ALL vs AML | 1.33 (1.27-1.39)† | 1.31 (1.23-1.40)† | 1.35 (1.25-1.45)† | 1.29 (1.22-1.35)† | 0.92 (0.89-0.96)† | 1.21 (1.12-1.30)† | 1.05 (0.99-1.12) |
| Age at least 42 y | 1.28 (1.22-1.34)† | 1.08 (1.01-1.15)‡ | 1.59 (1.47-1.70)† | 1.35 (1.28-1.41)† | 0.96 (0.92-0.99)‡ | 1.03 (0.95-1.11) | 1.16 (1.09-1.23)† |
| Female donor to male recipient vs other combination | 1.02 (0.97-1.08) | 0.95 (0.89-1.02) | 1.13 (1.04-1.22)‡ | 1.05 (0.99-1.11) | 0.92 (0.89-0.96)† | 1.12 (1.03-1.22)‡ | 1.28 (1.20-1.37)† |
| HLA-identical sibling vs other donor type | 0.89 (0.85-0.93)† | 1.10 (1.04-1.17)‡ | 0.66 (0.61-0.71)† | 0.83 (0.79-0.87)† | 1.20 (1.16-1.24)† | 0.82 (0.76-0.88)† | 0.98 (0.93-1.04) |
| RIC vs MAC | 1.04 (0.99-1.10) | 1.28 (1.20-1.38)† | 0.77 (0.71-0.85)† | 0.99 (0.93-1.04) | 0.97 (0.93-1.01) | 0.77 (0.70-0.84)† | 0.90 (0.84-0.97)‡ |
| CR vs other disease status | 0.50 (0.48-0.52)† | 0.45 (0.42-0.47)† | 0.58 (0.54-0.62)† | 0.49 (0.47-0.51)† | 1.05 (1.02-1.09)‡ | 0.90 (0.84-0.97)‡ | 0.91 (0.86-0.97)‡ |
| PB vs BM | 1.09 (1.04-1.15)‡ | 1.10 (1.03-1.18)‡ | 1.08 (1.00-1.17)‡ | 1.09 (1.04-1.15)‡ | 1.89 (1.82-1.97)† | 0.84 (0.78-0.91)† | 1.40 (1.31-1.50)† |
| Allo-SCT after the year 2004 | 0.89 (0.85-0.93)† | 0.95 (0.90-1.01) | 0.81 (0.75-0.87)† | 0.88 (0.84-0.92)† | 0.94 (0.91-0.97)‡ | 0.90 (0.83-0.97)‡ | 0.98 (0.93-1.04) |
| D-CMV+/R-CMV– vs D-CMV–/R-CMV– | 1.12 (1.04-1.21)‡ | 1.12 (1.01-1.24)‡ | 1.13 (1.00-1.28)‡ | 1.16 (1.07-1.26)‡ | 1.03 (0.97-1.10) | 1.10 (0.97-1.24) | 1.05 (0.95-1.15) |
| D-CMV–/R-CMV+ vs D-CMV–/R-CMV– | 1.10 (1.04-1.17)‡ | 1.03 (0.95-1.12) | 1.20 (1.09-1.32)‡ | 1.14 (1.07-1.21)‡ | 0.99 (0.94-1.03) | 0.97 (0.87-1.07) | 1.01 (0.93-1.10) |
| D-CMV+/R-CMV+ vs D-CMV–/R-CMV– | 1.13 (1.07-1.20)† | 1.08 (1.01-1.16)‡ | 1.21 (1.11-1.32)† | 1.15 (1.09-1.22)† | 1.00 (0.96-1.04) | 0.92 (0.85-1.01) | 0.98 (0.92-1.05) |
| D-CMV+/R-CMV+ vs D-CMV–/R-CMV+* | 0.97 (0.92-1.03) | 1.05 (0.97-1.13) | 1.00 (0.92-1.09) | 0.99 (0.93-1.05) | 0.99 (0.95-1.03) | 1.05 (0.95-1.15) | 1.03 (0.96-1.11) |
| D-CMV–/R-CMV– vs all other* | 0.89 (0.85-0.94)† | 0.93 (0.87-0.99)‡ | 0.84 (0.77-0.90)† | 0.87 (0.83-0.92)† | 1.00 (0.97-1.04) | 1.04 (0.96-1.13) | 1.00 (0.94-1.06) |
Shown are hazard ratios with 95% confidence interval (n = 16 628).
aGVHD, acute GVHD; cGVHD, chronic GVHD; NEG, neutrophil engraftment.
These comparisons have been done in separate multivariate Cox regression analyses, including all patient and transplant characteristics besides the CMV serostatus as specified in this table.
P < .0001.
P < .05.
Discussion
This study investigated the prognostic impact of donor/recipient CMV serostatus in 16 628 de novo acute leukemia patients after allo-SCT. Using univariate and multivariate analyses, D-CMV+ and/or R-CMV+ was associated with a significant decrease in LFS and OS, and an increase in NRM. These findings indicate that CMV serostatus still has an important prognostic impact in patients with acute leukemia who undergo allo-SCT, despite the wide implementation of monitoring and preemptive treatment of CMV in standard algorithms in recent years.1-3 Analyzing different causes of death that contribute to NRM, we further found that the 2-year probability of death as a result of infection was significantly increased in the case of D-CMV+ and/or R-CMV+. To obtain more insight into specific causes of death, we also analyzed the outcome in a subgroup of 7731 patients with documented CMV reactivation status (supplemental Table 6). Both LFS and OS were significantly reduced in the case of D-CMV+ and/or R-CMV+, even in patients who did not have any CMV reactivation until day +100 after allo-SCT. However, occurrence of CMV reactivation was also associated with a significantly reduced LFS and OS if it was taken as a time-dependent covariate. Together these observations confirm the previous hypothesis that the virus may have direct and indirect effects.2,13,14,21
The negative prognostic impact of D-CMV+ and/or R-CMV+ was apparently stronger in ALL than in AML patients, resulting in a 9% decline of 2-year OS in patients with ALL. Such impact on OS is within the range of other prognostic factors that are well established for this disease, such as patient’s age or leukocyte count at diagnosis.22 Furthermore, the decreases in LFS and OS in the case of D-CMV+ and/or R-CMV+ were accompanied by an increased RI and NRM in ALL patients. Conversely, the CMV serostatus likewise had a significant impact on NRM—but not RI—in AML patients. Because D-CMV+ and/or R-CMV+ belong to the most important risk factors for CMV reactivation, our findings argue against the so-called “CMV-vs-leukemia effect,” in particular in ALL patients.2,3,7-9 In addition, we analyzed the development of donor/recipient hematopoietic chimerism to elucidate further potential effects of the CMV serostatus on RI. D-CMV+ and/or R-CMV+ was associated with a significantly reduced frequency of complete hematopoietic donor/recipient chimerism in both ALL and AML patients (supplemental Table 7). The exact significance of this finding remains to be elucidated because the RI after allo-SCT is determined by many other variables in addition to hematopoietic chimerism.
In the literature, the influence of the CMV serostatus of the donor remains even more unclear than that of the recipient.12 A superior outcome for CMV-seropositive unrelated stem cell recipients with a CMV-seropositive vs a CMV-seronegative donor has been described in one study but not in the other; both studies included patients with different underlying disorders (eg, acute and chronic leukemia, aplastic anemia).15,16 In the present study, D-CMV+ vs D-CMV– was associated with a significantly decreased LFS and OS among CMV-seronegative recipients in multivariate analysis. However, the donor CMV serostatus had no prognostic impact among CMV-seropositive recipients in these analyses.
Scarce data are available regarding the impact of the CMV serostatus on engraftment and occurrence of GVHD.12,23,24 In the current study, the incidence of graft failure and time to neutrophil engraftment did not differ significantly when comparing D-CMV–/R-CMV– with other combinations of donor/recipient CMV serostatus. However, when analyzing patients separately according to diagnosis, D-CMV+ and/or R-CMV+ was associated with a slightly but statistically significant decreased 2-year cumulative incidence of neutrophil engraftment in ALL patients but not in AML patients. Published in vitro and in vivo data suggested that different herpes viruses—including CMV—might delay immunoreconstitution, which could explain these observations in ALL.21 Also, when comparing D-CMV–/R-CMV– with D-CMV+ and/or R-CMV+, we did not find a significant difference with regard to the occurrence of acute or chronic GVHD. Surprisingly, the risk of acute GVHD was significantly decreased in CMV-seropositive vs CMV-seronegative recipients. Taken together, these data further contradict the general understanding that CMV infection or disease—with R-CMV+ being one of the strongest risk factors for this complication—and GVHD may trigger each other in conjunction with a potential additional risk of GVHD because of tapering of immunosuppressive drugs after detection of CMV reactivation.12,23,24
Finally, diagnosis of AML vs ALL, younger age, use of an HLA-identical sibling donor, cytogenetic good risk, transplantation during first CR, allo-SCT after the year 2004, and a large center size (>50 transplants per year) were all associated with a significant superior LFS and OS in multivariate analysis. The plateau observed for both LFS and OS with 35% and 38%, respectively, at 10 years after allo-SCT suggests that de novo acute leukemia is cured by this approach in a significant proportion of patients.
In conclusion, this comprehensive analysis on the prognostic impact of donor/recipient CMV serostatus in de novo acute leukemia patients focused on the era of preemptive treatment of CMV infection showed that CMV serostatus still has an important prognostic impact on allo-SCT outcome despite the implementation of sophisticated techniques for monitoring and (preemptive) treatment of CMV in recent years.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all allogeneic transplantation centers of the EBMT group for reporting the data included in this analysis to this registry.
Authorship
Contribution: M.S.-H., M.L., E.P., I.W.B., and M.M. designed the research and/or analyzed data; D.B., L.V., G.E., J.F., G.S., R.S., N.K., A.G., D.N., and M.M. provided important clinical data; M.S.H. wrote the first draft of the manuscript; and all authors approved the final version of the manuscript.
A complete list of contributors, as well as members of the European Bone Marrow Transplantation Group appears in the online data supplement.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Schmidt-Hieber, HELIOS Clinic Berlin Buch, Clinic for Hematology, Oncology and Tumorimmunology, Schwanebecker Chaussee 50, 13175 Berlin, Germany; e-mail: martin.schmidt-hieber@helios-kliniken.de or martin.schmidt-hieber@charite.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal