Key Points
Decreased in vitro dose-response slope tracks with resistance BCR-ABL mutants to ABL tyrosine kinase inhibitors.
Integrating in vitro dose-response slope, the IC50 of various BCR-ABL mutants, and clinical PK data can predict CML patients’ response to TKIs.
Abstract
BCR-ABL mutations result in clinical resistance to ABL tyrosine kinase inhibitors (TKIs) in chronic myeloid leukemia (CML). Although in vitro 50% inhibitory concentration (IC50) values for specific mutations have been suggested to guide TKI choice in the clinic, the quantitative relationship between IC50 and clinical response has never been demonstrated. We used Hill’s equation for in vitro response of Ba/F3 cells transduced with various BCR-ABL mutants to determine IC50 and the slope of the dose-response curve. We found that slope variability between mutants tracked with in vitro TKI resistance, provides particular additional interpretive value in cases where in vitro IC50 and clinical response are disparate. Moreover, unlike IC50 alone, higher inhibitory potential at peak concentration (IPP), which integrates IC50, slope, and peak concentration (Cmax), correlated with improved complete cytogenetic response (CCyR) rates in CML patients treated with dasatinib. Our findings suggest a metric integrating in vitro and clinical data may provide an improved tool for BCR-ABL mutation-guided TKI selection.
Introduction
BCR-ABL kinase domain mutations represent a common mechanism of resistance to ABL tyrosine kinase inhibitors (TKIs) in chronic myeloid leukemia (CML). In vitro cellular 50% inhibitory concentration (IC50) values have been proposed to guide TKI treatment selection for specific mutations.1 However, using peak concentration (Cmax)/IC50 as a measure of potential in vivo activity failed to show a correlation with complete cytogenetic response (CCyR) rates in CML patients.2
Importantly, an IC50 value constitutes only one point on the dose-response curve for a given drug. Most dose-response curves can be described by Hill’s equation (equation 1), which incorporates both IC50 and slope (m) parameters:
Here, fa and fu are cell fractions affected and unaffected by treatment, respectively (fu = 1 − fa), and D is drug dose. Theoretical and clinical importance of evaluation of the slope in addition to IC50 has already been shown for antiretroviral drug resistance in HIV infection.3
We report an estimation of the slope of in vitro dose-response curves for wild-type and kinase domain–mutant BCR-ABL against clinical ABL TKIs for CML and examine the value of this incorporated parameter for predicting clinical response.
Methods
Ba/F3 cellular data
Dose-response curves for imatinib, nilotinib, and dasatinib were determined previously by methanethiosulfonate-based cell viability assay in Ba/F3 cells expressing wild-type or kinase domain–mutant BCR-ABL.4 Because it was completely insensitive to all 3 ABL TKIs tested, the BCR-ABLT315I mutant was excluded from our analysis.
Calculation of inhibitory potential values
Logarithmic transformation of the Hill’s equation reaches:
The parameters m and IC50 were determined for each mutation and drug by fitting equation (2) to the respective dose-response curve using the least-square-sum criterion. Inhibitory potential at peak concentration (IPP)3 was subsequently calculated as:
Here, D is mean Cmax in plasma as reported.2
Comparison with clinical response
IPP and IC50 values for each Ba/F3 BCR-ABL mutant were compared with previously reported CCyR rates for nilotinib5 and dasatinib.6 Response data for mutations reported in more than 2 patients was included, divided based on mutation IPP and IC50 values, and CCyR rates were compared between groups by 2-tailed Student t test with unequal variance (P = .05 significance threshold). Multivariate analysis was performed by linear multiple regression and the Cox proportional hazard model using JMP-SAS version 10 software (see supplementary material on the Blood Web site for details).
Results and discussion
We fitted Hill’s equation to Ba/F3 cell viability dose-response curves for imatinib, nilotinib, and dasatinib for wild-type BCR-ABL and each of 15 BCR-ABL kinase domain point mutants (see representative curves in supplemental Figure 1; all data reported in reference 4). Excellent goodness of fit (r2 values = 0.94-0.99) was observed for all drug-mutation pairings. Resultant values of IC50 and slope for each case are summarized in Table 1, along with calculated IPP values (see equation [3] in Methods). IPP provides a natural way to combine drug efficacy data in vitro (ie, IC50 and slope) with clinical pharmacokinetic data and compare them with clinical outcomes.
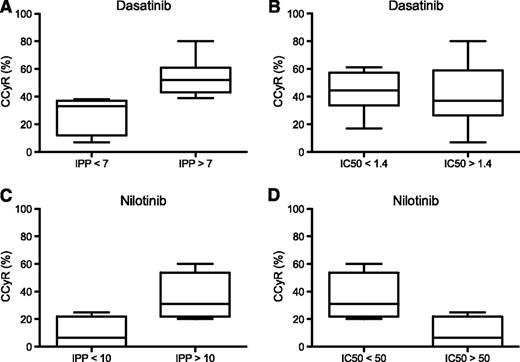
Correlation between IPP or IC50 and clinical response for dasatinib and nilotinib. IPP was calculated based on drug IC50 and slope of in vitro response of Ba/F3 cells expressing various BCR-ABL mutations and on population pharmacokinetic mean peak concentrations in plasma reported for each drug. Mutations were divided into 2 groups for dasatinib (A-B) and nilotinib (C-D) based on cutoff values of IPP (nondimensional) or IC50 (nM) as indicated. For each group, clinical response analysis was based on previously published mutation-specific rates of complete cytogenetic response (CCyR),5,6 and the median, range, and 25th and 75th percentiles are shown.
Correlation between IPP or IC50 and clinical response for dasatinib and nilotinib. IPP was calculated based on drug IC50 and slope of in vitro response of Ba/F3 cells expressing various BCR-ABL mutations and on population pharmacokinetic mean peak concentrations in plasma reported for each drug. Mutations were divided into 2 groups for dasatinib (A-B) and nilotinib (C-D) based on cutoff values of IPP (nondimensional) or IC50 (nM) as indicated. For each group, clinical response analysis was based on previously published mutation-specific rates of complete cytogenetic response (CCyR),5,6 and the median, range, and 25th and 75th percentiles are shown.
| . | Imatinib . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | WT . | M244V . | G250E . | Q252H . | Y253F . | Y253H . | E255K . | E255V . | F311L . | F317L . | M351T . | F359V . | V379I . | L387M . | H396P . | H396R . |
| R2 | 0.96 | 0.88 | 0.98 | 0.98 | 0.99 | 0.99 | 0.98 | 0.93 | 0.93 | 0.99 | 0.99 | 0.94 | 0.94 | 0.97 | 0.94 | 0.92 |
| Slope | 1.87 | 5 | 1.3 | 2 | 3.7 | 1.4 | 1.46 | 0.43 | 2.6 | 3.28 | 3.3 | 2.5 | 2.6 | 2.2 | 2.66 | 3.2 |
| IC50 | 274.5 | 1919.8 | 1184.3 | 854.1 | 3321.3 | 20508.0 | 5227.3 | 88907.2 | 419.2 | 982 | 942.5 | 897.8 | 1139.6 | 914.3 | 747.4 | 1096.6 |
| IPP | 4.82 | 3.19 | 1.66 | 2.93 | 0.85 | 0.084 | 0.46 | 0.22 | 5.59 | 4.28 | 4.43 | 3.50 | 3.04 | 3.06 | 4.20 | 3.83 |
| . | Imatinib . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | WT . | M244V . | G250E . | Q252H . | Y253F . | Y253H . | E255K . | E255V . | F311L . | F317L . | M351T . | F359V . | V379I . | L387M . | H396P . | H396R . |
| R2 | 0.96 | 0.88 | 0.98 | 0.98 | 0.99 | 0.99 | 0.98 | 0.93 | 0.93 | 0.99 | 0.99 | 0.94 | 0.94 | 0.97 | 0.94 | 0.92 |
| Slope | 1.87 | 5 | 1.3 | 2 | 3.7 | 1.4 | 1.46 | 0.43 | 2.6 | 3.28 | 3.3 | 2.5 | 2.6 | 2.2 | 2.66 | 3.2 |
| IC50 | 274.5 | 1919.8 | 1184.3 | 854.1 | 3321.3 | 20508.0 | 5227.3 | 88907.2 | 419.2 | 982 | 942.5 | 897.8 | 1139.6 | 914.3 | 747.4 | 1096.6 |
| IPP | 4.82 | 3.19 | 1.66 | 2.93 | 0.85 | 0.084 | 0.46 | 0.22 | 5.59 | 4.28 | 4.43 | 3.50 | 3.04 | 3.06 | 4.20 | 3.83 |
| . | Nilotinib . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | WT . | M244V . | G250E . | Q252H . | Y253F . | Y253H . | E255K . | E255V . | F311L . | F317L . | M351T . | F359V . | V379I . | L387M . | H396P . | H396R . |
| R2 | 0.99 | 0.99 | 0.99 | 0.97 | 0.97 | 0.94 | 0.98 | 0.94 | 0.98 | 0.99 | 0.99 | 0.99 | 0.94 | 0.96 | 0.99 | 0.99 |
| Slope | 2.1 | 5.64 | 1.66 | 0.94 | 4.06 | 1.13 | 3.1 | 0.98 | 3.08 | 2.88 | 2.84 | 2.72 | 2.56 | 1.58 | 2.5 | 2.95 |
| IC50 | 14.3 | 47.7 | 59.8 | 41.4 | 107.7 | 616.9 | 174.4 | 600.6 | 24.9 | 47.2 | 16.9 | 148.4 | 44.2 | 53.9 | 41.3 | 37.6 |
| IPP | 12.0 | 25.3 | 7.1 | 4.4 | 14.9 | 2.3 | 9.9 | 2.1 | 15.8 | 13.0 | 15.7 | 9.1 | 11.7 | 6.9 | 11.6 | 14.0 |
| CCyR% | 35 | 60 | 25 | 0 | 0 | 27 | 13 | 20 | ||||||||
| . | Nilotinib . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | WT . | M244V . | G250E . | Q252H . | Y253F . | Y253H . | E255K . | E255V . | F311L . | F317L . | M351T . | F359V . | V379I . | L387M . | H396P . | H396R . |
| R2 | 0.99 | 0.99 | 0.99 | 0.97 | 0.97 | 0.94 | 0.98 | 0.94 | 0.98 | 0.99 | 0.99 | 0.99 | 0.94 | 0.96 | 0.99 | 0.99 |
| Slope | 2.1 | 5.64 | 1.66 | 0.94 | 4.06 | 1.13 | 3.1 | 0.98 | 3.08 | 2.88 | 2.84 | 2.72 | 2.56 | 1.58 | 2.5 | 2.95 |
| IC50 | 14.3 | 47.7 | 59.8 | 41.4 | 107.7 | 616.9 | 174.4 | 600.6 | 24.9 | 47.2 | 16.9 | 148.4 | 44.2 | 53.9 | 41.3 | 37.6 |
| IPP | 12.0 | 25.3 | 7.1 | 4.4 | 14.9 | 2.3 | 9.9 | 2.1 | 15.8 | 13.0 | 15.7 | 9.1 | 11.7 | 6.9 | 11.6 | 14.0 |
| CCyR% | 35 | 60 | 25 | 0 | 0 | 27 | 13 | 20 | ||||||||
| . | Dasatinib . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | WT . | M244V . | G250E . | Q252 H . | Y253F . | Y253H . | E255K . | E255V . | F311L . | F317L . | M351T . | F359V . | V379I . | L387M . | H396P . | H396R . |
| R2 | 0.99 | 0.99 | 0.98 | 0.98 | 0.97 | 0.97 | 0.95 | 0.94 | 0.97 | 0.93 | 0.94 | 0.99 | 0.95 | 0.97 | 0.95 | 0.98 |
| Slope | 1.82 | 3.5 | 1.54 | 1.46 | 3.35 | 2.55 | 1.41 | 0.99 | 3.1 | 1.8 | 2.74 | 2.39 | 1.87 | 2.27 | 3.87 | 1.96 |
| IC50 | 1.2 | 1.3 | 1.5 | 1.2 | 1.2 | 1.1 | 5.6 | 11.3 | 1.1 | 6.2 | 1.0 | 1.8 | 1.5 | 1.5 | 1.8 | 1.1 |
| IPP | 7.8 | 14.8 | 6.4 | 6.4 | 14.5 | 11.3 | 3.9 | 2.2 | 13.7 | 4.8 | 12.3 | 9.4 | 7.6 | 9.2 | 15.1 | 8.7 |
| CCyR% | 56 | 43 | 33 | 17 | 61 | 38 | 36 | 7 | 46 | 52 | 8O | 39 | ||||
| . | Dasatinib . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | WT . | M244V . | G250E . | Q252 H . | Y253F . | Y253H . | E255K . | E255V . | F311L . | F317L . | M351T . | F359V . | V379I . | L387M . | H396P . | H396R . |
| R2 | 0.99 | 0.99 | 0.98 | 0.98 | 0.97 | 0.97 | 0.95 | 0.94 | 0.97 | 0.93 | 0.94 | 0.99 | 0.95 | 0.97 | 0.95 | 0.98 |
| Slope | 1.82 | 3.5 | 1.54 | 1.46 | 3.35 | 2.55 | 1.41 | 0.99 | 3.1 | 1.8 | 2.74 | 2.39 | 1.87 | 2.27 | 3.87 | 1.96 |
| IC50 | 1.2 | 1.3 | 1.5 | 1.2 | 1.2 | 1.1 | 5.6 | 11.3 | 1.1 | 6.2 | 1.0 | 1.8 | 1.5 | 1.5 | 1.8 | 1.1 |
| IPP | 7.8 | 14.8 | 6.4 | 6.4 | 14.5 | 11.3 | 3.9 | 2.2 | 13.7 | 4.8 | 12.3 | 9.4 | 7.6 | 9.2 | 15.1 | 8.7 |
| CCyR% | 56 | 43 | 33 | 17 | 61 | 38 | 36 | 7 | 46 | 52 | 8O | 39 | ||||
Imatinib
Most P-loop mutations are reported to render a worse response to imatinib.7 We found that 4 of 7 P-loop mutations tested (G250E, Y253H, E255K, E255V) showed a lower dose-response slope relative to wild-type BCR-ABL in addition to high IC50 (>1100 nM), whereas all other mutations showed variably increased slopes (Table 1). Consistent with particularly negative effects of these mutations on drug binding and clinical outcome with imatinib, their lower slopes indicate shallower drug efficacy over a given increase in concentration. Differences in slope values across different resistant mutations likely reflect a varied degree of inhibitor-binding destabilization (rather than binding preclusion). Furthermore, the range of IPPs for these mutations was lower than (and not overlapping with) all other mutations (0.084-1.66 vs 2.93-5.59; Student t test for range: P = 6×10−6).
Additional value of the slope parameter was particularly apparent in cases where increased in vitro IC50 does not track with clinical resistance. For example, in comparing the G250E and V379I mutants, which feature comparable cellular IC50 values for imatinib (1184 and 1140 nM, respectively), only G250E harbors worse clinical prognosis, arguably because of its lower dose-response slope. This is also reflected in a lower IPP value compared with V379I (Table 1). Similarly, the P-loop mutation M244V does not confer marked clinical resistance to imatinib despite increased IC50 relative to wild-type BCR-ABL, possibly because of an exceptionally high slope value (m = 5 vs m = 1.87 for wild-type BCR-ABL) reflecting a very steep dose-response curve (Table 1). Although at 6000 nM the IPP values for the M244V mutant and wild-type BCR-ABL become virtually identical (5.70 and 5.77, respectively), this concentration is below imatinib Cmax for some individuals but not for others,8 suggesting that patients with this mutation may be particularly vulnerable to consequences of unfavorable imatinib pharmacokinetic profile or reduced compliance. Indeed, one study found that clinical resistance to imatinib in 3 of 6 patients with a M244V mutation was overcome by dose increase.9
Nilotinib and dasatinib
Particularly high slope values (m>4) were found for M244V and Y253F with nilotinib treatment, in contrast to lower values for both mutations with dasatinib treatment (Table 1).
Comparison of BCR-ABL mutant IPP values between nilotinib and dasatinib revealed favorable, higher IPP for dasatinib over nilotinib against the Y253H mutant; nilotinib demonstrated higher IPP for E255K and F317L compared with dasatinib. For the E255V mutation, both drugs featured low IPPs, consistent with this mutation being reported in clinical failures of both drugs.5,6
We next examined whether higher IPP values are predictive of better clinical response. Patient response data were initially divided according to the median mutant IPP value for each TKI. Dasatinib-treated patients with mutations resulting in IPP values above the median (IPP = 8) had a significantly higher mean CCyR rate than patients with mutation IPPs below the median (53% [range, 39-80] vs 31% [range, 7-56]; P = .038). Notably, using an IPP threshold value of 7 resulted in even better group separation of mean CCyR rate (P = .007; Figure 1A and supplemental Figure 2 for P values for a range of threshold values). In contrast, this relationship was not evident for mean CCyR rates when IC50 alone was used as the comparator (P = .83 for IC50 values above vs below the median IC50; Figure 1B).
Upon comparison of nilotinib-treated patients by either mutation IPP or IC50 value, the difference in mean CCyR rate between patients with values above or below the median approached but did not reach statistical significance (P = .055 for both cases; Figure 1C-D). Notably, however, both the number of mutations and overall number of nilotinib-treated patients with published available mutation-response data were smaller than for dasatinib (7 vs 11 mutations, 65 vs 295 patients with mutations, respectively).
Multivariate analysis by 2 methods also demonstrated that a model including IPP and TKI type significantly correlates with CCyR (P < .001) and outperforms a model that includes IC50 and slope as separate variables based on the model significance, covariate significance, and Akaike information criterion (supplemental Table 1).
In conclusion, we show that in vitro dose-response slope provides important additional information regarding efficacy of ABL TKIs beyond in vitro IC50 values: lower slopes are indicative of increased resistance, and very high slopes result in a steep dose-response curve, presenting potential challenges in cases of low compliance or unfavorable pharmacokinetic profile. Calculation of IPP (based on IC50, slope, and clinical plasma Cmax) allows for separation of patients with respect to rates of CCyR with dasatinib, unlike using in vitro IC50 alone.2 Further implementation of our results for improvement of mutationally-guided TKI treatment selection in CML will require incorporation of additional variables such as plasma protein binding, drug clearance and distribution, and validation using independent large patient databases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr Ilan Roziner for his help with statistical analysis of the data.
Authorship
Contribution: V.V. and B.J.D. designed research; T.O. and B.J.D. contributed all in vitro experimental data; V.V. and C.A.E. performed the research; O.S. performed statistical analysis; and V.V., C.A.E., and B.J.D. wrote the manuscript.
Conflict-of-interest disclosure: V.V. is employed by Neumedicines, Inc., as a clinical advisor. B.J.D. is currently principal investigator or co-investigator on Novartis and Bristol-Myers Squibb clinical trials. B.J.D.’s institution has contracts with these companies to pay for patient costs, nurse and data manager salaries, and institutional overhead. B.J.D. does not derive salary, nor does his laboratory receive funds, from these contracts. Oregon Health & Science University (OHSU) and B.J.D. have a financial interest in MolecularMD. OHSU has licensed technology used in some of these clinical trials to MolecularMD. B.J.D.’s potential individual and institutional conflict of interest has been reviewed and managed by OHSU.
Correspondence: Vladimir Vainstein, Department of Hematology, Hadassah Medical Center, Ein Kerem, Jerusalem, Israel; e-mail: vladimir.dr@gmail.com.



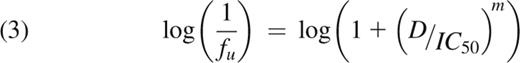
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal