Key Points
HVLL is a chronic EBV+ lymphoproliferative disorder of childhood with risk to develop systemic lymphoma.
The disease shows favorable response to conservative therapy despite the presence of a T- or NK-cell monoclonal proliferation.
Abstract
Hydroa vacciniforme–like lymphoma (HVLL) is an Epstein-Barr virus (EBV)-positive T-cell lymphoproliferative disorder of childhood that occurs mainly in Central and South America and Asia. We present the clinicopathological features of 20 Mexican children with HVLL with a median age of 8 years at diagnosis (range, 1-15). All patients presented with skin lesions involving sun-exposed areas, but not exclusively. Fever, lymphadenopathy, and hepatosplenomegaly were often observed. Most patients were treated with immunomodulators and/or immunosuppressive agents, resulting in temporary remission. For 13 patients follow-up was available for a median of 3 years (range, 1 month-13 years). Three patients with long follow-up (9-13 years) are alive with disease. Four patients died, 2 after developing systemic lymphoma. Histologically, the skin showed a predominantly angiocentric and periadnexal Epstein-Barr early RNA+ lymphoid infiltrate with variable atypia and subcutaneous involvement. Fifteen patients showed a T-cell phenotype (12, αβ; 2, γδ; 1, silent phenotype) and monoclonal T-cell receptor-γ rearrangements, whereas 6 exhibited a natural killer (NK)-cell phenotype. Four patients had hypersensitivity to mosquito bites. One patient showed both phenotypes. HVLL is an EBV-associated lymphoproliferative disorder of αβ-, γδ-, or NK-cell phenotype with a broad clinical spectrum, usually prolonged clinical course, and risk for progression to systemic disease.
Introduction
In its classic form, hydroa vacciniforme (HV) is characterized by light-induced vesicles that evolve to crusts and leave varicelliform scars after healing. Systemic symptoms are not observed, and the disease usually remits spontaneously in adolescence or young adulthood.1 In past years a peculiar group of vesicopapular eruptions that mimic HV was recognized in children mainly from Mexico,2,3 Peru,4 and Asia.5-8 These lesions present with marked facial edema, vesicles, crusts, and large ulcers, with severe scarring and disfigurement in sun-exposed and nonexposed skin areas. The patients usually have systemic symptoms including fever, weight loss, and asthenia. Hepatosplenomegaly and lymphadenopathy are frequently observed in the acute phase, and association with hypersensitivity to mosquito bites (HMB) was noted.2,9 Because panniculitis and/or vasculitis were the predominant histological features, initially it was suggested that this condition be called edematous scarring vasculitic panniculitis (ESVP) to separate it from the classic form of HV.2 Later studies demonstrated that these lesions, which were termed “severe” HV or ESVP, were associated with Epstein-Barr virus (EBV) infection and often showed monoclonal rearrangements of the T-cell receptor (TCR) genes; therefore, the term “HV-like lymphoma” was suggested.3,10
HV-like lymphoma (HVLL) and systemic EBV-positive T-cell lymphoproliferative disease (LPD) of childhood were incorporated for the first time in the 2008 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues in the subgroup of EBV-positive T-cell LPD of childhood.11 HVLL is defined as an EBV-positive cutaneous T-cell lymphoma that occurs in children and less often in young adults12 and may be associated with hypersensitivity to insect bites. Clinically, patients present with facial edema and recurrent vesiculopapular rash followed by ulceration and crusting. Although the skin lesions are not exclusively limited to sun-exposed areas and do not only appear after sun exposure, as do HV skin lesions, there is a seasonal increased occurrence during the summer. Most cases reveal a CD8+ T-cell phenotype;4 however, a small proportion of cases have been reported to have a natural killer (NK)-cell phenotype.9,13-15 In contrast, only rare cases of CD4+ T-cell phenotype have been described.16,17 The lymphoid cells, regardless of cell-type derivation, are positive for cytotoxic markers such as granzyme B and T-cell intracellular antigen 1 (TIA-1).4,16 CD30 expression was found in a portion of the reported cases in the literature.3
Since the incorporation of HVLL into the WHO lymphoma classification, several controversial issues have been raised that remain to be clarified. It is not known whether HVLL represents a true lymphoma from its first clinical manifestation or a preneoplastic disorder with risk of developing into a systemic lymphoma.2,7 It is also uncertain whether HVLL is a de novo disease3 or develops in patients with long-standing HV, as has been suggested.15,18 Classic HV in Western countries is considered a benign photodermatosis with spontaneous improvement or remission during adolescence. These cases are rarely biopsied; in the original study, clonality and EBV status were not investigated.1,19,20 Subsequent studies in Asian populations showed that “classic” HV was also an EBV-associated disorder. Therefore, it was proposed that it be included as part of the clinical spectrum of chronic active EBV infection.10,15,21 Nevertheless, it is unclear whether what has been called “classic” HV in Asian populations corresponds to the same disease described in Western populations and/or Mexico, where the disease is self-limited and progression to HVLL has not been observed.2 This discrepancy has contributed to uncertainty in the differential diagnosis between classic HV and HVLL. It has been proposed that the most useful criterion to separate these 2 entities is monoclonality of the TCR genes.4,21,22 Nevertheless, the differential diagnosis seems to be arbitrary, posing serious diagnostic and therapeutic problems for pathologists, dermatologists, and hematologists.
A related cutaneous disorder, mainly described in Japan, is severe mosquito bite allergy.23 Severe mosquito bite allergy is defined as an EBV+ NK-cell lymphoproliferation characterized by high fever, ulcers, skin necrosis, and deep scarring after bites, with the potential to progress into overt lymphoma or leukemia in the long-standing clinical course.24 Severe mosquito bite allergy is not recognized as a distinct entity in the 2008 WHO classification, and its relationship to HVLL is not well defined. Accumulating evidence indicates that these 2 cutaneous disorders might represent different manifestations within the spectrum of disorders encompassed under the umbrella of chronic active EBV infection of T/NK-cell type.21
The aim of this study was to analyze the clinical, histological, and molecular features of 20 Mexican children who were diagnosed, treated, and followed at the Instituto Nacional de Pediatria in Mexico City, Mexico, with the diagnosis of ESVP or HVLL. In addition, we aimed to define more precisely the diagnostic criteria and prognosis of the disease in order to facilitate the development of more effective treatments. Our study reveals that EBV+ HV-like LPD, regardless of the presence or absence of systemic symptoms and the severity of the skin lesions, is a monoclonal disorder of T cells and/or NK cells with a broad clinical spectrum, a usually protracted clinical course, and long-term risk to progress to a systemic lymphoma.
Material and methods
Case selection
Twenty-eight biopsies from 20 Mexican children with the diagnosis of ESVP, from which tissue blocks were available for EBV in situ hybridization (ISH) and immunohistochemical and molecular analyses, were included in the study. Cases were collected from the files of the Department of Pathology, Instituto Nacional de Pediatria, Mexico City (17 cases), from 1976 to 2009, and from the Department of Pathology, Instituto Nacional de Ciencias Medicas y de la Nutricion Salvador Zubiran (3 cases). Some cases have been previously reported;2 however, the biopsies were completely and independently analyzed in the current study. Clinical information and follow-up were obtained from patient records or treating physicians. The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the National Institute of Pediatria in Mexico. The cases belong to a local disease registry.
Immunohistochemistry and EBV ISH
The morphological and immunohistochemical features were analyzed on formalin-fixed and paraffin-embedded tissue sections. Immunohistochemical stains were performed using an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer’s protocol. The following panel of antibodies was used: CD3 (clone SP7; DCS, Hamburg, Germany; dilution 1:100), CD4 (clone SP35; Zytomed, Berlin, Germany), CD8 (clone C8/144B; Dako, Glostrup, Denmark), CD30 (clone Ber-H2; Dako), CD56 (clone 123C3; Dako), LMP-1 (clone CS 1-4; Dako), TIA-1 (clone 4i389; Zytomed), β F1 (Thermo Scientific, Fremont, CA), TCR-gamma (Thermo Scientific), and EBV nuclear antigen 2 (EBNA-2) antibody (clone PE2; Novocastra, Berlin, Germany).
All cases were subjected to ISH using oligonucleotides complementary to EBER transcripts in paraffin-embedded tissue in an automated stainer (Ventana Medical Systems). Double staining for EBER ISH and immunohistochemistry was performed following the above-described methods. ISH was performed first without counterstain, followed by immunohistochemistry in an automated immunostainer.25
Polymerase chain reaction analyses of TCR-γ and -δ gene rearrangements
T-cell clonality was analyzed by polymerase chain reaction (PCR) amplification of the TCR-γ chain genes with 2 distinct protocols according to McCarthy26 and Trainor27 with some modifications. DNA used for PCR was extracted from 10-µm paraffin sections after dewaxing and proteinase K digestion applying standard phenol/chloroform purification procedures. Two PCRs of TCR-γ rearrangements were performed in duplicates (30 ng and 100 ng DNA) using Phusion Hot Start DNA polymerase with Phusion GC buffer containing magnesium chloride and 30 ng and 100 ng of DNA, respectively. Selected samples were analyzed in duplicate for TCR-δ rearrangements using the commercially available BIOMED-2 assay28 (InVivoScribe Technologies, Inc., San Diego, CA) according to the manufacturer’s instructions. The products were separated by capillary electrophoresis on the GenomeLab GeXP Genetic Analysis System and analyzed by the GenomeLab GeXP software 10.2 (Beckman Coulter, Brea, CA).
Results
Clinical findings
Clinical features of the 20 patients are summarized in Table 1. The group consisted of 14 males and 6 females (ratio 2.3:1) with a median age at the time of diagnosis of 8 years (range, 1-15 years). The mean duration of disease at the time of consultation was 2.4 years (1-5 years). All patients presented with a dermatosis characterized by vesicles, blisters, erythema, ulcerations, crusts, and vacciniforme scars involving mainly the face and ear lobes (Figure 1A-B). Facial and hand edema was a prominent feature. Patients often presented with severe, deep skin lesions with disfiguring scars in both covered and sun-exposed areas (Figure 1C-D). Light avoidance did not prevent the development of skin lesions. None of the patients had light hypersensitivity. Three patients were clinically diagnosed with cutaneous lymphoma of childhood, bullous urticarial, or psoriasiform dermatitis. Ten patients (50%) presented with disseminated dermatosis and systemic symptoms such as fever, wasting, lymphadenopathy, and hepatosplenomegaly. These symptoms were often observed in patients with severe cutaneous lesions. Ten patients (50%) presented with only skin lesions without systemic symptoms. In 4 patients HMB was documented. Follow-up was available for 13 patients with a median age of 3 years (range, 1 month-13 years). However, 5 of these patients were lost during follow-up (1-24 months), mostly after treatment with good response (cases 6, 10, 12, 13, and 14). Three patients (cases 1, 4, and 15) with long follow-up (9-13 years) are alive with waxing and waning disease. Four patients died, 2 after developing systemic lymphoma (2-4 years), including a sinonasal NK/T-cell lymphoma and a peripheral T-cell lymphoma, not otherwise specified (PTCL nos). One patient died secondary to hepatic failure (case 9) without active skin lesions. No autopsy was performed. One patient died of complications of chemotherapy (case 20). Seven patients were lost to follow-up after skin biopsies were taken and a diagnosis was rendered.
Clinical data of 20 Mexican children with HVLL
| Case . | Sex . | Age . | Clinical diagnosis . | Symptoms at diagnosis . | Treatment . | Follow-up . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Edema . | Blisters . | Ulcers . | Crusts . | Scars . | FTTH + HS . | ||||||
| 1a | M | 10 | ESVP | Thalidomide, topical steroids | 9 y | ||||||
| 1b | 16 | 3 y history | − | + | − | + | + | + | Remission and recurrences AwD | ||
| 2 | F | 13 | Lymphoma | − | + | + | − | − | − | — | LFU |
| 3 | M | 4 | EVSP | — | LFU | ||||||
| 2 y history | − | + | − | + | + | + | |||||
| 4a | M | 11 | HVLL | Thalidomide, topical steroids | 13 y | ||||||
| 4b | 20 | − | + | − | + | + | + | Chemotherapy | Remission and recurrences | ||
| 4c | 24 | AwD | |||||||||
| 5 | M | 1 | Bullous urticaria | − | + | − | + | + | − | — | LFU |
| 6 | F | 6 | ESVP | Chloroquine | 5 mo improvement | ||||||
| 1.5 y history | + | + | + | + | + | + | LFU | ||||
| 7 | M | 13 | ESVP | − | + | + | — | LFU | |||
| 8a | F | 10 | ESVP | Cyclophosphamide systemic steroids | 3 y remission and recurrences | ||||||
| 8b | 13 | Vasculitis, HMB | − | + | + | + | + | + | LFU | ||
| 9 | M | 6 | ESVP | Thalidomide, systemic steroids, cyclophosphamide | 3 y improved | ||||||
| 2 mo | + | + | + | + | + | + | DwD* | ||||
| 10 | M | 8 | Psoriasiform dermatitis/ESVP | Systemic steroids, IFN | 13 mo improved | ||||||
| 12 mo history | − | + | − | + | + | + | LFU | ||||
| 11 | F | 11 | ESVP 2 y history | Thalidomide | 4 y improved | ||||||
| 15 | PTCL, nos | + | + | + | + | + | − | — | LFU/DoD | ||
| 12 | M | 15 | ESVP | Thalidomide, | 2 y Improved | ||||||
| 12 mo history | + | − | + | + | + | − | Topical steroids, chloroquine | LFU | |||
| 13 | M | 8 | ESVP | Thalidomide, systemic steroids | 1 mo improved | ||||||
| 5 y history | + | + | + | + | + | − | LFU | ||||
| 14 | M | 12 | ESVP | Thalidomide, systemic steroids, cyclophosphamide | 2 mo improved | ||||||
| 18 mo history | − | + | + | + | + | − | LFU | ||||
| 15° | M | 6 | ESVP | Thalidomide, systemic steroids, methrotexate, cyclophosphamide | 12 y | ||||||
| 15b | 7 | + | + | + | + | + | + | AwD | |||
| 15c | 12 | ||||||||||
| 16° | M | 8 | ESVP | Thalidomide | 4 y | ||||||
| 16b | 12 | Nasal NK/T-cell lymphoma | + | + | + | + | + | + | Chemotherapy | DoD | |
| 16c | 12 | ||||||||||
| 17 | F | 6 | ESVP, HMB | — | LFU | ||||||
| 4 y history | − | + | + | + | + | − | |||||
| 18 | F | 6 | ESVP | + | + | + | + | + | − | — | LFU |
| 19 | M | 8 | ESVP, HMB | — | LFU | ||||||
| 3 y history | + | + | + | + | + | − | |||||
| 20 | M | 15 | HVLL, HMB | Chemotherapy | 2 mo | ||||||
| 2 y history | − | + | + | + | + | − | DoD | ||||
| Case . | Sex . | Age . | Clinical diagnosis . | Symptoms at diagnosis . | Treatment . | Follow-up . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Edema . | Blisters . | Ulcers . | Crusts . | Scars . | FTTH + HS . | ||||||
| 1a | M | 10 | ESVP | Thalidomide, topical steroids | 9 y | ||||||
| 1b | 16 | 3 y history | − | + | − | + | + | + | Remission and recurrences AwD | ||
| 2 | F | 13 | Lymphoma | − | + | + | − | − | − | — | LFU |
| 3 | M | 4 | EVSP | — | LFU | ||||||
| 2 y history | − | + | − | + | + | + | |||||
| 4a | M | 11 | HVLL | Thalidomide, topical steroids | 13 y | ||||||
| 4b | 20 | − | + | − | + | + | + | Chemotherapy | Remission and recurrences | ||
| 4c | 24 | AwD | |||||||||
| 5 | M | 1 | Bullous urticaria | − | + | − | + | + | − | — | LFU |
| 6 | F | 6 | ESVP | Chloroquine | 5 mo improvement | ||||||
| 1.5 y history | + | + | + | + | + | + | LFU | ||||
| 7 | M | 13 | ESVP | − | + | + | — | LFU | |||
| 8a | F | 10 | ESVP | Cyclophosphamide systemic steroids | 3 y remission and recurrences | ||||||
| 8b | 13 | Vasculitis, HMB | − | + | + | + | + | + | LFU | ||
| 9 | M | 6 | ESVP | Thalidomide, systemic steroids, cyclophosphamide | 3 y improved | ||||||
| 2 mo | + | + | + | + | + | + | DwD* | ||||
| 10 | M | 8 | Psoriasiform dermatitis/ESVP | Systemic steroids, IFN | 13 mo improved | ||||||
| 12 mo history | − | + | − | + | + | + | LFU | ||||
| 11 | F | 11 | ESVP 2 y history | Thalidomide | 4 y improved | ||||||
| 15 | PTCL, nos | + | + | + | + | + | − | — | LFU/DoD | ||
| 12 | M | 15 | ESVP | Thalidomide, | 2 y Improved | ||||||
| 12 mo history | + | − | + | + | + | − | Topical steroids, chloroquine | LFU | |||
| 13 | M | 8 | ESVP | Thalidomide, systemic steroids | 1 mo improved | ||||||
| 5 y history | + | + | + | + | + | − | LFU | ||||
| 14 | M | 12 | ESVP | Thalidomide, systemic steroids, cyclophosphamide | 2 mo improved | ||||||
| 18 mo history | − | + | + | + | + | − | LFU | ||||
| 15° | M | 6 | ESVP | Thalidomide, systemic steroids, methrotexate, cyclophosphamide | 12 y | ||||||
| 15b | 7 | + | + | + | + | + | + | AwD | |||
| 15c | 12 | ||||||||||
| 16° | M | 8 | ESVP | Thalidomide | 4 y | ||||||
| 16b | 12 | Nasal NK/T-cell lymphoma | + | + | + | + | + | + | Chemotherapy | DoD | |
| 16c | 12 | ||||||||||
| 17 | F | 6 | ESVP, HMB | — | LFU | ||||||
| 4 y history | − | + | + | + | + | − | |||||
| 18 | F | 6 | ESVP | + | + | + | + | + | − | — | LFU |
| 19 | M | 8 | ESVP, HMB | — | LFU | ||||||
| 3 y history | + | + | + | + | + | − | |||||
| 20 | M | 15 | HVLL, HMB | Chemotherapy | 2 mo | ||||||
| 2 y history | − | + | + | + | + | − | DoD | ||||
AwD, alive with disease; DoD, died of disease: DwD, died without disease; ESVP, edematous, scarring vasculitic panniculitis; FTTH, failure to thrive; HS, hepatosplenomegaly; IFN, interferon; LFU, lost to follow-up.
Died of hepatic failure, no active skin lesions.
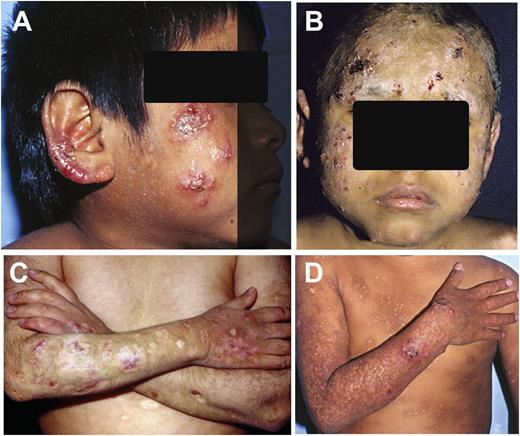
Skin lesions in HVLL. (A-B) Multiple skin lesions in face and ear lobes characterized by vesicles, erythema, and crusts. (C-D) In some cases, the lesions produce extensive tissue loss and disfigurement (C) and/or vacciniforme scars (D) in sun-exposed areas.
Skin lesions in HVLL. (A-B) Multiple skin lesions in face and ear lobes characterized by vesicles, erythema, and crusts. (C-D) In some cases, the lesions produce extensive tissue loss and disfigurement (C) and/or vacciniforme scars (D) in sun-exposed areas.
Histological and immunophenotypical features
Twenty-eight biopsies from 20 patients were analyzed. All biopsies showed similar histological findings, characterized by a lymphoid infiltrate predominantly in the dermis that sometimes extended deep into the subcutaneous tissue (Figure 2A). The infiltrate was mainly located around adnexae and blood vessels, often with angiodestructive features. The intensity of the infiltrate and atypia of the lymphocytes varied from case to case. There were biopsies with relatively few reactive-appearing lymphocytes (Figure 2B), biopsies with dense infiltrate (Figure 2C), and obvious cytological atypia characterized by large cells with irregular nuclei, prominent nucleoli, and abundant clear cytoplasm. In 11 of the 20 biopsies an intraepidermal spongiotic vesicle was observed without epidermotropism (Figure 2D).
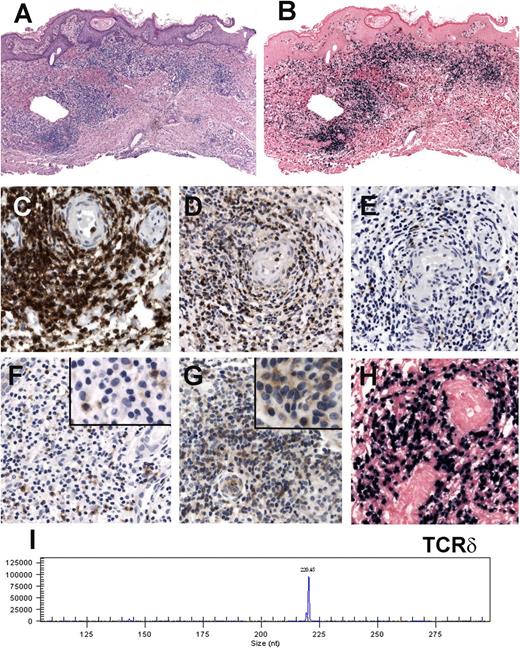
HVLL morphology and immunophenotype. (A-B) Case 1: Skin biopsy showing an infiltrate mainly in the dermis that extends into the subcutaneous fat. Note that the infiltrate surrounds adnexae (hematoxylin and eosin [H&E] stain, original magnification ×25). (B) Higher magnification shows a small lymphoid infiltrate without atypia surrounding a blood vessel (H&E stain, original magnification ×400). (C) Case 15: Skin biopsy showing a dense infiltrate with atypical medium to large lymphoid cells with rather abundant cytoplasm (H&E stain, original magnification ×400). Insert: The atypical cells have irregular nuclei with large eosinophilic nucleoli (H&E stain, original magnification ×630). (D) Case 11: Skin biopsy with intraepidermal bullae and a dense infiltrate in the upper dermis surrounding adnexae and blood vessels (H&E stain, original magnification ×100). Immunohistochemical analysis in a case with a αβ T-cell phenotype (case 9). (E) CD3 stain shows that the cells surrounding the adnexa are strongly CD3 positive. Additionally, the infiltrating cells are TIA-1 positive (F), CD8 positive (G), and negative for CD4 (H; E-H, immunoperoxidase, original magnification ×200). (I) CD30 staining reveals many positive cells (immunoperoxidase, original magnification ×400). (J) EBER ISH is positive in the infiltrating lymphocytes. Note that the number of EBER-positive cells is less than those positive for CD3 (original magnification ×200).
HVLL morphology and immunophenotype. (A-B) Case 1: Skin biopsy showing an infiltrate mainly in the dermis that extends into the subcutaneous fat. Note that the infiltrate surrounds adnexae (hematoxylin and eosin [H&E] stain, original magnification ×25). (B) Higher magnification shows a small lymphoid infiltrate without atypia surrounding a blood vessel (H&E stain, original magnification ×400). (C) Case 15: Skin biopsy showing a dense infiltrate with atypical medium to large lymphoid cells with rather abundant cytoplasm (H&E stain, original magnification ×400). Insert: The atypical cells have irregular nuclei with large eosinophilic nucleoli (H&E stain, original magnification ×630). (D) Case 11: Skin biopsy with intraepidermal bullae and a dense infiltrate in the upper dermis surrounding adnexae and blood vessels (H&E stain, original magnification ×100). Immunohistochemical analysis in a case with a αβ T-cell phenotype (case 9). (E) CD3 stain shows that the cells surrounding the adnexa are strongly CD3 positive. Additionally, the infiltrating cells are TIA-1 positive (F), CD8 positive (G), and negative for CD4 (H; E-H, immunoperoxidase, original magnification ×200). (I) CD30 staining reveals many positive cells (immunoperoxidase, original magnification ×400). (J) EBER ISH is positive in the infiltrating lymphocytes. Note that the number of EBER-positive cells is less than those positive for CD3 (original magnification ×200).
The immunohistochemical findings are summarized in Table 2. In all 28 biopsies the infiltrate was predominantly CD3+ with very few scattered CD20+ cells. The cytotoxic marker TIA-1 was positive in all biopsies analyzed. In 11 of 20 patients (55%), the initial biopsy showed a proliferation of cytotoxic TCR-αβ T cells with expression of CD8+, β F1+, and TIA-1+ and negativity for CD4 and CD56 (Figure 2E-H). Two cases (10%; cases 6 and 13) were double negative for CD4/CD8 and β F1 but positive for TCR-γ, which is indicative of a γδ T-cell derivation (Figure 3A-G). One of these cases was focally positive for CD56, and one case (case 10) was TCR silent. In 5 cases the CD3+TIA-1+ cells were double negative for CD4/CD8, β F1 negative, and homogeneously strong for CD56+, which is indicative of an NK-cell phenotype (Figure 4A-E). The lymphoid infiltrate in the cases with an NK-cell phenotype tended to involve the subcutaneous tissue, with rimming of the neoplastic cells surrounding individual fat cells, mimicking subcutaneous panniculitic T-cell lymphoma (SPTCL; Figure 4B). Three of the NK-cell cases revealed numerous eosinophils scattered within the lymphoid infiltrate, all had a history of HMB (Figure 4F). In case 15, 3 biopsies were available. At diagnosis the lymphoid infiltrate was composed of cytologically bland cells (Figure 4I); however, the atypia of the infiltrating cells increased in the follow-up biopsies 1 and 7 years after the original diagnosis (Figure 4J-K). In case 20, the infiltrating lymphocytes showed expression of CD8, β F1, and CD56 (supplemental Figure 1). The lymphoid infiltrate was intermingled with abundant eosinophils. Eleven cases (55%) showed a variable number of CD30+ cells (Figure 4G). Few scattered CD4+ reactive lymphocytes were observed in all cases analyzed. LMP1 was positive only in 3 cases (Figure 4H), whereas EBNA-2 remained negative in all cases analyzed. CD30 expression was more frequently found in biopsies with an NK-cell phenotype (6 of 7; 86%) than in biopsies with a T-cell phenotype (6 of 13; 46%).
Immunophenotype and molecular analysis of 20 cases of HVLL
| Case . | CD3 . | CD4 . | CD8 . | TIA-1 . | CD56 . | CD30 . | BF1 . | EBER . | LMP . | EBNA2 . | TCR-δ . | TCR-γ . | Lineage . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | + | ND | + | ND | − | + | ND | + | ND | ND | ND | Mono | T-cell αβ |
| 1b | + | − | + | ND | − | ND | + | ND | ND | ND | |||
| 2 | + | − | + | + | − | − | ND | + | ND | ND | Poly | Mono | T-cell αβ |
| 3 | + | ND | + | ND | ND | − | ND | + | ND | ND | ND | Mono | T-cell αβ |
| 4a | + | − | + | + | − | − | + | + | − | − | ND | ND | |
| 4b | + | − | + | + | − | ND | + | + | − | − | Poly | Mono | T-cell αβ |
| 4c | + | − | + | + | − | ND | + | + | − | − | Poly | Mono | |
| 5 | + | − | + | + | − | + | + | + | − | − | ND | Mono | T-cell αβ |
| 6 | + | − | − | + | − | − | −* | + | − | − | Mono | Mono | T-cell γδ |
| 7 | + | − | + | + | − | − | + | + | − | − | ND | Mono | T-cell αβ |
| 8a | + | − | + | + | −/+ | + | + | + | − | − | Poly | Mono | T-cell αβ |
| 8b† | + | − | −/+ | + | + | + | −/+ | + | − | Poly | Mono | NK | |
| 9 | + | − | + | + | − | + | + | + | − | − | ND | ND | T-cell αβ |
| 10 | + | − | −/+ | + | −/+ | + | − | + | − | − | Poly | Poly | silent |
| 11 | + | − | + | + | − | + | + | + | + | − | Poly | Mono | T-cell αβ |
| 12 | + | − | + | + | − | − | + | + | − | − | ND | Mono | T-cell αβ |
| 13 | + | − | −/+ | + | + | − | −* | + | − | − | Mono | Mono | T-cell γδ |
| 14 | + | − | + | + | − | + | + | + | + | − | Poly | Mono | T-cell αβ |
| 15a | + | − | − | + | + | + | − | + | ND | ND | ND | Poly | NK |
| 15b‡ | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 15c | + | − | − | + | + | + | − | + | − | − | |||
| 16a | + | − | − | + | + | + | − | + | ND | ND | ND | Poly | NK |
| 16b§ | + | − | − | + | + | + | − | + | ND | ND | |||
| 16c | + | − | − | + | + | + | − | + | ND | ND | |||
| 17† | + | − | − | + | + | + | − | + | − | − | Poly | Poly | NK |
| 18 | + | − | − | + | + | + | − | + | + | − | Poly | Poly | NK |
| 19† | + | − | − | + | + | − | − | + | − | − | Poly | Poly | NK |
| 20† | + | − | +/− | + | +/− | + | +/− | + | − | − | Poly | Mono | T-cell αβ? |
| Case . | CD3 . | CD4 . | CD8 . | TIA-1 . | CD56 . | CD30 . | BF1 . | EBER . | LMP . | EBNA2 . | TCR-δ . | TCR-γ . | Lineage . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | + | ND | + | ND | − | + | ND | + | ND | ND | ND | Mono | T-cell αβ |
| 1b | + | − | + | ND | − | ND | + | ND | ND | ND | |||
| 2 | + | − | + | + | − | − | ND | + | ND | ND | Poly | Mono | T-cell αβ |
| 3 | + | ND | + | ND | ND | − | ND | + | ND | ND | ND | Mono | T-cell αβ |
| 4a | + | − | + | + | − | − | + | + | − | − | ND | ND | |
| 4b | + | − | + | + | − | ND | + | + | − | − | Poly | Mono | T-cell αβ |
| 4c | + | − | + | + | − | ND | + | + | − | − | Poly | Mono | |
| 5 | + | − | + | + | − | + | + | + | − | − | ND | Mono | T-cell αβ |
| 6 | + | − | − | + | − | − | −* | + | − | − | Mono | Mono | T-cell γδ |
| 7 | + | − | + | + | − | − | + | + | − | − | ND | Mono | T-cell αβ |
| 8a | + | − | + | + | −/+ | + | + | + | − | − | Poly | Mono | T-cell αβ |
| 8b† | + | − | −/+ | + | + | + | −/+ | + | − | Poly | Mono | NK | |
| 9 | + | − | + | + | − | + | + | + | − | − | ND | ND | T-cell αβ |
| 10 | + | − | −/+ | + | −/+ | + | − | + | − | − | Poly | Poly | silent |
| 11 | + | − | + | + | − | + | + | + | + | − | Poly | Mono | T-cell αβ |
| 12 | + | − | + | + | − | − | + | + | − | − | ND | Mono | T-cell αβ |
| 13 | + | − | −/+ | + | + | − | −* | + | − | − | Mono | Mono | T-cell γδ |
| 14 | + | − | + | + | − | + | + | + | + | − | Poly | Mono | T-cell αβ |
| 15a | + | − | − | + | + | + | − | + | ND | ND | ND | Poly | NK |
| 15b‡ | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 15c | + | − | − | + | + | + | − | + | − | − | |||
| 16a | + | − | − | + | + | + | − | + | ND | ND | ND | Poly | NK |
| 16b§ | + | − | − | + | + | + | − | + | ND | ND | |||
| 16c | + | − | − | + | + | + | − | + | ND | ND | |||
| 17† | + | − | − | + | + | + | − | + | − | − | Poly | Poly | NK |
| 18 | + | − | − | + | + | + | − | + | + | − | Poly | Poly | NK |
| 19† | + | − | − | + | + | − | − | + | − | − | Poly | Poly | NK |
| 20† | + | − | +/− | + | +/− | + | +/− | + | − | − | Poly | Mono | T-cell αβ? |
αβ?, probably αβ lineage; Mono, monoclonal; ND, not done; Poly, polyclonal; −/+, a minority of cells; +/−, many cells; +, most cells.
Positive for TCR-γ by immunohistochemistry.
History of HMB.
Only hematoxylin and eosin stain available.
Nasal mucosa biopsy.
Immunohistochemical analysis, EBER ISH, and molecular analysis in a case with γδ T-cell phenotype (case 6). (A) Skin biopsy showing a dense lymphoid infiltrate in the dermis surrounding blood vessels and adnexae (hematoxylin and eosin stain, original magnification ×100). (B) EBER ISH reveals that practically all lymphoid cells are EBER positive (original magnification ×100). (C-G) The cells are CD3 (C) and TIA-1 positive (D) but negative for CD8 (E) and β F1 (F). Note that the lymphoid cells are TCR-γ positive (G) (C-G: immunoperoxidase, original magnification ×400). (Inset F-G: immunoperoxidase, original magnification ×630). (H) Higher magnification of the EBER ISH (original magnification ×400). (I) TCR-δ gene rearrangement shows a dominant monoclonal peak of 220 base pairs.
Immunohistochemical analysis, EBER ISH, and molecular analysis in a case with γδ T-cell phenotype (case 6). (A) Skin biopsy showing a dense lymphoid infiltrate in the dermis surrounding blood vessels and adnexae (hematoxylin and eosin stain, original magnification ×100). (B) EBER ISH reveals that practically all lymphoid cells are EBER positive (original magnification ×100). (C-G) The cells are CD3 (C) and TIA-1 positive (D) but negative for CD8 (E) and β F1 (F). Note that the lymphoid cells are TCR-γ positive (G) (C-G: immunoperoxidase, original magnification ×400). (Inset F-G: immunoperoxidase, original magnification ×630). (H) Higher magnification of the EBER ISH (original magnification ×400). (I) TCR-δ gene rearrangement shows a dominant monoclonal peak of 220 base pairs.
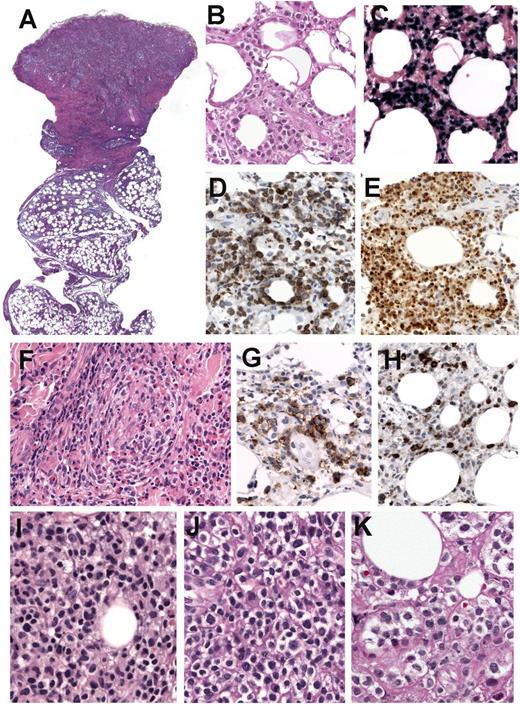
Immunohistochemical analysis and EBER ISH in a case with NK-cell phenotype (case 15). (A) Hematoxylin and eosin (H&E) stain of a skin biopsy with a dense infiltrate extending from the upper dermis deep into the subcutaneous tissue (original magnification ×12.5). (B-E) Higher magnification shows atypical lymphoid cells with rimming of individual fat cells mimicking subcutaneous panniculitis-like T-cell lymphoma (B). The cells are EBER positive (C), strongly and homogeneously CD56 positive (D), and TIA-1 positive (E). (B-E: original magnification ×400). (F) Higher magnification of the dermis reveals angioinvasion with abundant eosinophils (original magnification ×400). (G) CD30 stain shows many positive cells. (H) LMP1 is positive only in a few scattered cells (G-H: immunohistochemistry, original magnification ×400). (I-K) Comparative morphology of 3 skin biopsies in case 15. (I) Skin biopsy at diagnosis shows a lymphoid infiltrate of rather small lymphocytes without atypia. (J) Skin biopsy 1 year after diagnosis shows a lymphoid infiltrate composed of medium-sized cells with abundant clear cytoplasm. (K) Skin biopsy 7 years after the original diagnosis with rather atypical large cells, abundant clear cytoplasm, and prominent nucleoli (I-K: H&E stain, original magnification ×400).
Immunohistochemical analysis and EBER ISH in a case with NK-cell phenotype (case 15). (A) Hematoxylin and eosin (H&E) stain of a skin biopsy with a dense infiltrate extending from the upper dermis deep into the subcutaneous tissue (original magnification ×12.5). (B-E) Higher magnification shows atypical lymphoid cells with rimming of individual fat cells mimicking subcutaneous panniculitis-like T-cell lymphoma (B). The cells are EBER positive (C), strongly and homogeneously CD56 positive (D), and TIA-1 positive (E). (B-E: original magnification ×400). (F) Higher magnification of the dermis reveals angioinvasion with abundant eosinophils (original magnification ×400). (G) CD30 stain shows many positive cells. (H) LMP1 is positive only in a few scattered cells (G-H: immunohistochemistry, original magnification ×400). (I-K) Comparative morphology of 3 skin biopsies in case 15. (I) Skin biopsy at diagnosis shows a lymphoid infiltrate of rather small lymphocytes without atypia. (J) Skin biopsy 1 year after diagnosis shows a lymphoid infiltrate composed of medium-sized cells with abundant clear cytoplasm. (K) Skin biopsy 7 years after the original diagnosis with rather atypical large cells, abundant clear cytoplasm, and prominent nucleoli (I-K: H&E stain, original magnification ×400).
ISH and molecular analysis
The results of EBER ISH and TCR-γ and -δ clonality analyses are summarized in Table 2. ISH for EBV using EBER1 probe showed numerous EBER+ cells in all cases. However, in most cases the amount of EBER+ cells represented only a subpopulation of the CD3+ infiltrating cells (Figure 2J). The amount of EBER+ cells was similar in cases with T- and NK-cell phenotypes. EBER+ cells concentrated mainly around the blood vessels and adnexae in the dermis (Figures 2 and 3), in the subcutaneous tissue (Figure 4C), and in the basal epithelial layer in cases with intraepidermal vesicles. In case 20, due to the expression of CD8 and CD56 in the lymphoid infiltrate, double stainings with EBER and CD8 or CD56 were performed. EBER+ cells were predominantly CD56+, whereas few cells were CD8+, suggesting an NK-cell derivation of the infiltrating cells (supplemental Figure 1). However, molecular analysis revealed a monoclonal rearrangement of the TCR-γ genes favoring a T-cell derivation. Due to the latter finding, the case was classified as likely being of T-cell phenotype, although the presence of 2 EBV-infected populations cannot be excluded. Case 8 had 2 biopsies; the first showed a predominantly CD8+/EBER+ dermal infiltrate. However, the second biopsy, taken 3 years later, showed a more panniculitic infiltrate with predominance of CD56+/EBER+ cells and with very few CD8+ cells (supplemental Figure 2). This case demonstrates that both EBV+ T- and NK-cell populations can occur in the same patient.
Molecular analysis demonstrated monoclonal rearrangement of the TCR-γ genes in 13 of the 14 cases with a T-cell phenotype. In the 2 cases with TCR-γ expression (cases 6 and 13), a monoclonal TCR-δ gene rearrangement was also demonstrated, further supporting the γδ T-cell derivation of these 2 cases. In 2 cases with several biopsies (cases 4 and 8), clonality analyses showed the same monoclonal TCR-γ gene rearrangement in the 2 biopsies taken several years apart (4 and 3 years apart, respectively), despite the subtle infiltrate found in subsequent biopsies (Figure 5; supplemental Figure 2). The 5 cases with an NK-cell phenotype (cases 15-19) showed a polyclonal rearrangement of the TCR-γ and -δ genes.
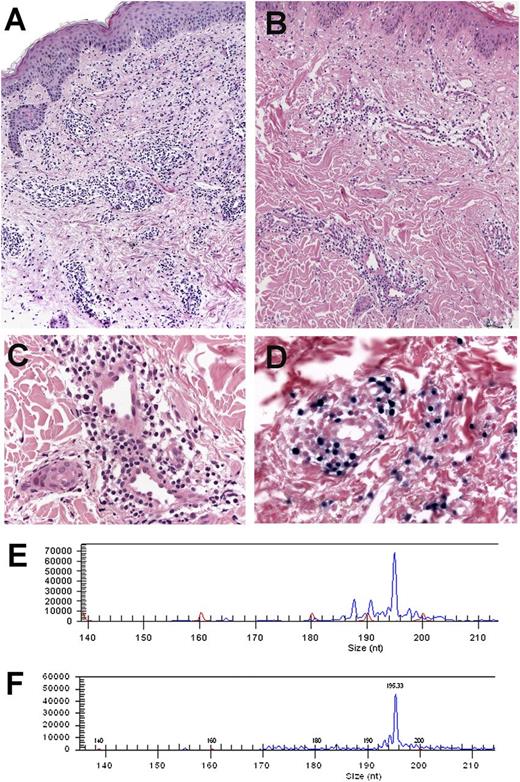
Morphology and comparative TCR-γ clonality analysis in case 4. (A-B) Hematoxylin and eosin stain in 2 recurrent skin biopsies taken 4 years apart at the age of 20 and 24 years. Note that the first biopsy (A) shows a denser lymphoid infiltrate when compared with the second biopsy (B; A-B: original magnification ×100). (C) Higher magnification of the second biopsy reveals a discrete infiltrate surrounding blood vessels with bland morphology. (D) Some of the lymphoid cells are EBER positive (C-D: original magnification ×400). (E-F) Amplification of the CD3 region of the TCR-γ chain gene showed an identical monoclonal peak of 195 base pairs in both biopsies.
Morphology and comparative TCR-γ clonality analysis in case 4. (A-B) Hematoxylin and eosin stain in 2 recurrent skin biopsies taken 4 years apart at the age of 20 and 24 years. Note that the first biopsy (A) shows a denser lymphoid infiltrate when compared with the second biopsy (B; A-B: original magnification ×100). (C) Higher magnification of the second biopsy reveals a discrete infiltrate surrounding blood vessels with bland morphology. (D) Some of the lymphoid cells are EBER positive (C-D: original magnification ×400). (E-F) Amplification of the CD3 region of the TCR-γ chain gene showed an identical monoclonal peak of 195 base pairs in both biopsies.
Treatment
Due to the long study period, patients received a variety of treatments. However, 13 patients were primarily treated with immunomodulating or immunosuppressive therapy, such as thalidomide, steroids, and/or chloroquine. The skin lesions usually improved with these treatments, and even though new skin lesions usually recurred, the amount of infiltrate and EBV+ cells, in general, decreased in follow-up biopsies (Figure 5). The 3 patients with the longest follow-up (9, 12, and 13 years) were treated with a combination of thalidomide and steroids repeatedly during acute flare-ups of their disease. At some point, 2 patients (cases 4 and 15) received additional chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) with only a transient effect. Due to side effects, both patients stopped chemotherapy and continued their treatment with thalidomide and steroids with relatively good response. In case 8, due to an initial diagnosis of vasculitis, the patient was treated with cyclophosphamide and prednisone with good results after 3 years of follow-up. Two patients (cases 11 and 16) developed systemic lymphoma while being treated with thalidomide, both died of disease. Patient 20 was treated with CHOP because of the diagnosis of HVLL. He had a 2-year history of skin lesions before receiving chemotherapy. The patient died of infectious complications while receiving chemotherapy.
Differences between patients with a T-cell and NK-cell phenotype
We compared the clinical features between cases with a T-cell phenotype and those with an NK-cell phenotype. The male-to-female ratio was similar in both groups (T cell: 2.5:1 vs NK cell: 2:1). The median age at consultation tended to be younger in patients with an NK-cell phenotype (8 years vs 10 years). The skin lesions in both groups were very similar; however, patients with a T-cell phenotype more often had systemic symptoms at presentation (57% vs 33%). HMB was documented in 3 patients with an NK-cell phenotype and in 1 patient with a T-cell phenotype and expression of CD56 (case 20). Progression to NK-/T-cell lymphoma manifested in the nasal region occurred in 1 of 3 patients with NK-cell phenotype for whom follow-up was available, whereas only 1 of 6 patients with a T-cell phenotype developed a PTCL nos.
Discussion
HVLL is a rare LPD originally described in Mexican children as ESVP.2 In the first study, it was described as a benign but clinically severe systemic disease with malignant potential that was not related to classic HV. Because subsequent studies demonstrated that these lesions often showed monoclonal rearrangements of the TCR genes, the term “HVLL” was proposed.3,4 The 2008 WHO classification recognized the increasing awareness of EBV-associated monoclonal disorders of T-cell or NK-cell origin in children and young adults and, for the first time, incorporated the group of childhood EBV-positive T-cell LPD into the lymphoma classification.11 In this study, we investigated the clinicopathological features of 20 Mexican children diagnosed with HVLL. The severity of the skin lesions and the clinical presentation varied among the patients and showed a broad spectrum. In contrast to classic HV, the lesions were larger and deeper and, in some cases, produced extensive tissue loss and disfigurement and were not associated with light hypersensitivity. Systemic symptoms such as fever, lymphadenopathy, and/or hepatosplenomegaly were common, especially in patients with severe cutaneous lesions and a T-cell phenotype. Central to the disease was the relatively long clinical course before patients sought medical attention (range, 1-5 years), underlining the chronic nature of the disorder. Here we demonstrate that the infiltrating EBV+ lymphocytes can consist of T cells either of αβ or γδ derivation or NK cells. Occasionally, coexistence of more than 1 phenotype of EBV+-infected cells can be observed. Regardless of the severity of the skin lesions and the presence or absence of systemic symptoms, cases with a T-cell phenotype constantly showed monoclonal rearrangement of the TCR-γ genes. Furthermore, in 2 cases with long follow-up and several biopsies, the same T-cell clone was demonstrated several years apart, indicating that monoclonality and clonal persistence are not predictive of an aggressive disease or of a progressive clinical course. Accordingly, in a recent report from Japan, Kimura et al24 reported 4 cases of “classic” HV, defined as patients with a characteristic dermatosis without systemic symptoms or cellular atypia. Nevertheless, all 4 cases were reclassified as HVLL based on the monoclonality of the TCR-γ genes. Our study and those of others15,29 suggest that EBV+ HV-like lesions are often monoclonal in nature and have overlapping histological features regardless of the presence or absence of systemic symptoms. Furthermore, no difference in the amount of infiltrating EBER+ cells has been found between these disorders.15
Recently it has been reported that HV and/or HVLL are associated with increased numbers of EBV-infected γδ T cells in peripheral blood.24,30,31 Kimura et al24 demonstrated that the EBV-infected cells in peripheral blood in almost half of HVLL patients (6 of 13 cases) were γδ T cells. Nevertheless, in none of these studies was the phenotype of the skin-infiltrating cells described. In our study, the vast majority of cases with a T-cell phenotype (12 of 15 cases) showed a αβ phenotype, whereas only a minority (2 of 15) were of γδ derivation. It is not clear whether the difference in the prevalence of EBV+ γδ T cells between the Japanese population and ours is related to racial differences or only reflects discrepancies between the EBV-infected cells in the peripheral blood and the cells infiltrating the skin.
Another interesting aspect of this study is that 30% of all HVLL cases revealed an NK-cell phenotype, similar to the Peruvian study32 and the 38% recently reported by the Japanese group,24 indicating that a third of all HVLL are of NK-cell phenotype, which is more than previously observed. Patients with NK-cell phenotype tended to have more “panniculitic” lesions with increased amounts of infiltrating eosinophils, often associated with HMB. Morphologically, these lesions can mimic SPTCL, primary cutaneous γδ T-cell lymphoma, or cutaneous involvement by an extranodal NK/T-cell lymphoma of the nasal type. Without clinical information, the differential diagnoses with the latter might be impossible to resolve because the morphology and phenotype of the EBV+-infiltrating cells are indistinguishable. Our study confirms previous results that indicated that HMB is usually associated with a proliferation of EBV-infected NK cells.13,23,33 Interestingly, patients with NK-cell phenotype in this study rarely presented with systemic symptoms despite sometimes alarming histology. Accordingly, previous studies have shown that patients with NK-cell phenotype show a relatively indolent clinical course when compared with patients with a T-cell phenotype.9,23 Nevertheless, patients with an NK-cell phenotype seem to have a higher risk of developing a systemic lymphoma, such as aggressive NK-cell leukemia or extranodal NK-cell lymphoma, nasal type, during the long clinical course.24 The severity of the clinical presentation has been proposed to prognosticate which cases will eventually progress to a systemic disease. In the study by Iwatsuki et al,15 5 of 11 patients diagnosed with severe HV developed NK-/T-cell lymphoma 2 to 14 years after the onset of disease. Of note, all cases were associated with NK-cell lymphocytosis, HMB, and/or hemophagocytosis. Two of these cases were classified as “subcutaneous lymphomas” without further specifications. This raises the possibility that these lesions represented further manifestations of the disease and not a progression to a systemic lymphoma. In this study, only 2 patients, 1 with a T-cell phenotype and 1 with an NK-phenotype, developed a systemic lymphoma relatively soon after the initial diagnosis (2 and 4 years, respectively). However, long clinical follow-up was available only for 3 patients, 2 with a T-cell phenotype and 1 with an NK-cell phenotype.
Although HVLL is characterized by a monoclonal proliferation of T cells or NK cells, the best approach for treatment remains uncertain. Chemotherapy and/or radiotherapy have been used in many patients but have been shown to be of little or no benefit. The effect is usually transient and does not induce sustained remission in most cases.4,24 Furthermore, patients receiving chemotherapy have been reported to have a worse prognosis with short survival.14 In the study by Barrionuevo et al,4 5 of 8 patients who received chemotherapy died primarily due to sepsis and liver failure with only slight improvement of the skin lesions. Similar results were reported by Rodriguez-Pinilla et al,32 where 8 of 11 patients who received chemotherapy and/or radiotherapy died, 5 were secondary to infectious complications. Accordingly, in this study 1 patient died of infectious complications while receiving chemotherapy. In contrast, immunomodulating therapies, such as prednisolone, cyclosporine A,24 interferon α,14 chloroquine, and thalidomide,2 have been shown to result in temporary remission or improvement of symptoms. These results indicate that a conservative approach should be recommended as first-line therapy in these patients.
In conclusion, HVLL is considered an EBV+ cutaneous T-cell lymphoma; this is based solely on the demonstration of a monoclonal T-cell proliferation. However, the long waxing and waning clinical course and the relatively good response to immunomodulating therapy challenge the concept of a full-blown malignant lymphoma at onset. Criteria such as presence of systemic symptoms, T-cell clonality, amount of EBV+ cells, and/or density of the infiltrate do not help in predicting which patients will eventually progress to systemic disease. Our data support the concept that EBV+ HV-like lesions represent different clinical severities of the same disease within the spectrum of EBV-associated cutaneous LPD. In order to avoid unnecessarily aggressive treatment and the stigma of a lymphoma diagnosis, the term “HV-like EBV+ LPD” to encompass the different clinical manifestations of the EBV-associated HV-like cutaneous lesions both of T-cell and NK-cell origin would be preferable for clinical usage. The challenge remains to identify morphological or clinical markers to predict which patients are at risk of progressing to a systemic lymphoma.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Annemarie Adam, Claudia Kloß, and Sema Colak for excellent technical assistance.
This study was supported in part by a grant from the Deutsche Forschungsgemeinschaft (SFB-685) to L.Q.-M. and F.F.
Authorship
Contribution: L.Q.-M. and F.F. designed the study, analyzed data, and wrote the manuscript; C.R. provided samples and helped with writing the manuscript; F.N., G.A., and P.G. performed the pathology work; I.B. performed and analyzed the molecular analysis; and M.S.-d.-O., C.D.-M., R.R.-M., and C.L.-M. provided samples and clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leticia Quintanilla-Martínez, Institute of Pathology and Comprehensive Cancer Center, University of Tübingen, Liebermeisterstr. 8, D-72076 Tübingen, Germany; e-mail: leticia.quintanilla-fend@med.uni-tuebingen.de.

![Figure 2. HVLL morphology and immunophenotype. (A-B) Case 1: Skin biopsy showing an infiltrate mainly in the dermis that extends into the subcutaneous fat. Note that the infiltrate surrounds adnexae (hematoxylin and eosin [H&E] stain, original magnification ×25). (B) Higher magnification shows a small lymphoid infiltrate without atypia surrounding a blood vessel (H&E stain, original magnification ×400). (C) Case 15: Skin biopsy showing a dense infiltrate with atypical medium to large lymphoid cells with rather abundant cytoplasm (H&E stain, original magnification ×400). Insert: The atypical cells have irregular nuclei with large eosinophilic nucleoli (H&E stain, original magnification ×630). (D) Case 11: Skin biopsy with intraepidermal bullae and a dense infiltrate in the upper dermis surrounding adnexae and blood vessels (H&E stain, original magnification ×100). Immunohistochemical analysis in a case with a αβ T-cell phenotype (case 9). (E) CD3 stain shows that the cells surrounding the adnexa are strongly CD3 positive. Additionally, the infiltrating cells are TIA-1 positive (F), CD8 positive (G), and negative for CD4 (H; E-H, immunoperoxidase, original magnification ×200). (I) CD30 staining reveals many positive cells (immunoperoxidase, original magnification ×400). (J) EBER ISH is positive in the infiltrating lymphocytes. Note that the number of EBER-positive cells is less than those positive for CD3 (original magnification ×200).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/18/10.1182_blood-2013-05-502203/4/m_3101f2.jpeg?Expires=1765888962&Signature=clBf1xl5VoiA30OkLZxnez7I8vgBLgv~CUgiXv~Qx4WCwYIQ6nMTsAmxfwgu2slWxKpszrWd8CtkLqjh9Xreqo5QTmv99QGdvRZv7swuRcATsLu37CILsWF-65GV7NIpxAo2mIb~yrvim38oTfn2EnJC5qR2L9Ioyux7Aev2~h~AoRdkGaqg3aPiZWSLnjpFgn0KoOn0HDYg7pKK0uJ5GsZpUMdqI1Tu~nNdK0tL~HByhTpwJJDbbXzCDXhHlgqkaVp0cziXJSDzjFWzNiEpKJdYiIBlwVILpZueTAfEli53MgDsIk8TqiL3ND3bRTS6t1QyWmds29hzji~VwJYFog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)