To the editor:
The virtually 100% diagnostic sensitivity and specificity of the BRAF V600E mutation for hairy cell leukemia (HCL), within the context of mature B-cell neoplasms, originally described by Tiacci et al,1 was recently confirmed in Blood.2,3 We report this mutation in a well-documented unclassifiable splenic B-cell lymphoma/leukemia, a mimic of HCL, illustrating a potential diagnostic pitfall.
A 36-year-old woman presented initially with cytopenias (white blood cell count [WBC] 2.5, 47% neutrophils, 50% lymphocytes, and 3% monocytes; hemoglobin [Hb] 12.1; and platelets 72) and splenomegaly (19 cm). A peripheral blood smear was not evaluated. Bone marrow demonstrated a CD5– CD20+CD23+κ+ B-cell infiltrate involving 60% of the specimen in an interstitial pattern. Rituximab for 2 years led to a minor response, and the patient went without therapy for 1.5 years. She subsequently re-presented with worsening cytopenias (WBC 2.0, Hb 11.1, and platelets 30) and abdominal bloating. Imaging demonstrated increased splenomegaly (26 cm) with enlarged para-aortic nodes. Splenectomy was performed.
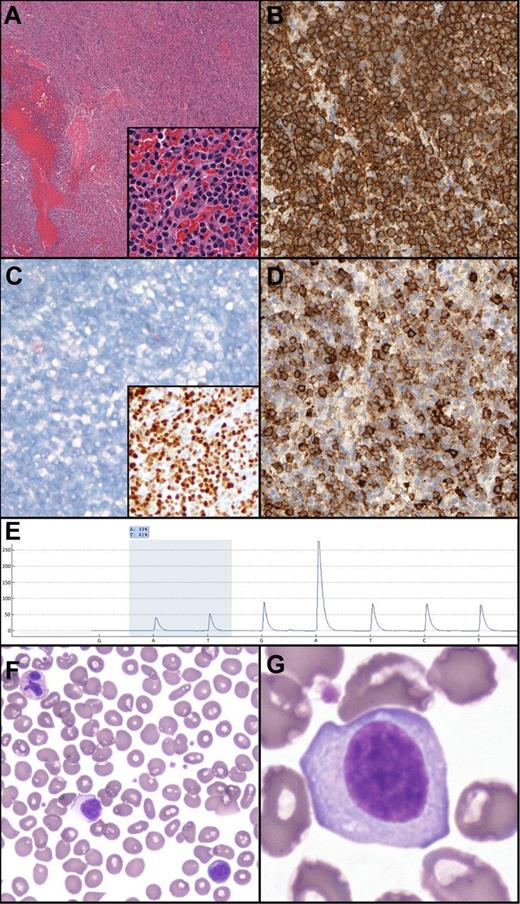
Splenic histology demonstrated architecture diffusely effaced by small mature lymphocytes with mildly irregular nuclear contours, absent nucleoli, and moderate amounts of pale cytoplasm (Figure 1). No targetoid patterning was present; indeed, the white pulp was essentially absent. Flow cytometry demonstrated κ+CD19+CD20(bright)+CD11c+CD23+CD25– CD103– B-cells. By immunohistochemistry, the B-cells were Annexin 1– and DBA44(subset)+.4
Unclassifiable splenic B-cell lymphoma/leukemia: histologic, morphologic, immunohistochemical and genetic features. (A) Hematoxylin and eosin demonstrates a diffuse splenic infiltrate of small mature lymphocytes, atrophic/absent white pulp, and occasional blood lakes. The infiltrate is composed of mature lymphocytes (inset). (B) CD20 expression in the neoplastic lymphocytes. (C) Absence of Annexin 1 (main panel) and positive p-ERK (inset) in the neoplastic B cells. (D) Subset DBA.44 expression in neoplastic B cells. (E) Pyrogram demonstrating the BRAF c.1799T>A mutation (shaded area; percentage indicates relative abundance). Nucleotide dispensation order is indicated along the x-axis. (F-G) Peripheral blood smear demonstrating atypical lymphocytes with abundant cytoplasm, absent villous cytoplasmic extensions, and inconspicuous nucleoli.
Unclassifiable splenic B-cell lymphoma/leukemia: histologic, morphologic, immunohistochemical and genetic features. (A) Hematoxylin and eosin demonstrates a diffuse splenic infiltrate of small mature lymphocytes, atrophic/absent white pulp, and occasional blood lakes. The infiltrate is composed of mature lymphocytes (inset). (B) CD20 expression in the neoplastic lymphocytes. (C) Absence of Annexin 1 (main panel) and positive p-ERK (inset) in the neoplastic B cells. (D) Subset DBA.44 expression in neoplastic B cells. (E) Pyrogram demonstrating the BRAF c.1799T>A mutation (shaded area; percentage indicates relative abundance). Nucleotide dispensation order is indicated along the x-axis. (F-G) Peripheral blood smear demonstrating atypical lymphocytes with abundant cytoplasm, absent villous cytoplasmic extensions, and inconspicuous nucleoli.
Pyrosequencing demonstrated a BRAF V600E mutation in 39% of analyzed splenic DNA. This mutation was confirmed by massively parallel sequencing (allele frequency 35.4%) using multiplexed, paired end reads on the Illumina MiSeq platform. Positive phosphorylated extracellular signal-regulated kinase (p-ERK) immunohistochemistry confirmed activation of the rapidly accelerated fibrosarcoma (RAF)–mitogen-activated protein kinase/ERK kinase (MEK)–ERK pathway.5,6
A complete blood count several weeks post splenectomy demonstrated an improvement in counts (WBC 6.4, 60% lymphocytes, 38% neutrophils, 2% monocytes; Hb 11.8; and platelets 341). Concurrent peripheral blood smear review demonstrated a subpopulation of atypical lymphocytes with abundant cytoplasm, absent villous cytoplasmic extensions, and inconspicuous nucleoli.
The features are diagnostic of a splenic lymphoma/leukemia, unclassifiable.7 Lack of CD25, CD103, and Annexin 1 expression (among other features) precludes a diagnosis of HCL or HCL-variant, and the absence of villous projections on circulating lymphocytes, non-intrasinusoidal bone marrow infiltration, and monocytopenia exclude splenic diffuse red pulp small B-cell lymphoma.
In addition to HCL, BRAF V600E mutations have also been described in other mature B-cell lymphoproliferative disorders,2 chronic lymphocytic leukemia (CLL),5,8 and B-prolymphocytic leukemia (PLL).8 However, these have primarily been detected by sensitive allele-specific polymerase chain reaction (consistent with minor subclones); only 1 case each of CLL and PLL has been confirmed with Sanger sequencing. Additionally, a different BRAF mutation (K601E) confirmed via Sanger sequencing was identified in a splenic marginal zone lymphoma.9 BRAF V600E mutations have not been previously reported in unclassifiable splenic B-cell lymphoma/leukemias.3 The case presented herein represents the first confirmed BRAF V600E mutation in this neoplasm and reinforces the notion that the BRAF V600E mutation is not synonymous with HCL. This finding has biological and therapeutic implications and highlights the need for additional genetic interrogation in classifying splenic B-cell lymphomas.
Authorship
Contribution: P.W.R. and A.B. wrote the manuscript; D.M. and M.H. provided clinical and pathologic case materials; M.O.N., J.J.D.M., and R.D. performed essential assays; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adam Bagg, Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, 7.103 Founders Pavilion, 3400 Spruce St, Philadelphia, PA 19104-4283; e-mail: adambagg@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal